(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
No fee required. | |||||
o | Fee computed on table below per Exchange Act Rules 14a-6(i) | ||||
| 1) | Title of each class of securities to which transaction applies: | ||||
| 2) | Aggregate number of securities to which transaction applies: | ||||
| 3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | ||||
| 4) | Proposed maximum aggregate value of transaction: | ||||
| 5) | Total fee paid: | ||||
o | Fee paid previously with preliminary | ||||
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the | ||||
| 1) | Amount previously paid: | ||||
| 2) | Form, Schedule or Registration Statement No.: | ||||
| 3) | Filing Party: | ||||
| 4) | Dated Filed: | ||||
|

PROXY STATEMENT
AND NOTICE OF ANNUAL
SHAREHOLDER MEETING

PLEASE VIEW OUR 2019 ANNUAL REPORT
investor.regeneron.com/2019AR

PLEASE VIEW OUR 2019 RESPONSIBILITY REPORT
investor.regeneron.com/2019RR



LETTER TO SHAREHOLDERS
DEAR FELLOW SHAREHOLDERS,
As 2020 began, the world was presented with a major crisis as the SARS-CoV-2 (COVID-19) pandemic spread across the globe. Regeneron mobilized quickly, realizing that we were uniquely suited to bring forward potential solutions. We are extremely proud of how our talented team is responding to this public health challenge. Given our more than 30 years of investment in core technologies that improve the drug discovery and development process, and our track record of success with infectious diseases like Ebola, we are optimistic that we can make a meaningful and timely impact. We have two important COVID-19 research and development efforts ongoing: a global clinical trial of our IL-6 inhibitor Kevzara®(sarilumab) in hospitalized patients with severe or critical COVID-19; and the development of a novel antibody cocktail specifically designed to prevent or treat COVID-19 infection. We will be sharing updates on these programs as quickly as possible over the coming weeks and months.
Now, more than ever, we must remain focused on our mission to repeatedly bring important new medicines to patients with serious diseases. In 2019 we had a year of strong performance and delivery on this mission. We reached more and more patients through newly-approved indications and record growth for our blockbuster treatments EYLEA®(aflibercept) Injection and Dupixent®(dupilumab), made significant advancements throughout our preclinical and clinical pipelines, and delivered a public health breakthrough with an effective treatment for Ebola which is currently under review by the U.S. Food and Drug Administration.
Total revenues for 2019 were $7.9 billion, a 17 percent increase over 2018, which included U.S. EYLEA net product sales of $4.6 billion. Our collaborator Bayer recorded net product sales for EYLEA outside the U.S. of $2.9 billion, bringing EYLEA’s total global net product sales to $7.5 billion, a 12 percent increase compared to 2018. These figures show the continuing strength of this important treatment that has had double-digit sales growth for seven years without a single price increase. We remain confident in EYLEA’s ability to help even more patients as we expand our leadership position in wet age-related macular degeneration and diabetic eye diseases. Dupixent 2019 global net product sales, which are recorded by Sanofi, were $2.3 billion, an increase of 151 percent over the previous year. We have just begun to tap the potential of this first-in-class treatment option for several Type 2 inflammatory diseases, with many more studies underway.
In part due to Dupixent’s strong sales, our antibody collaboration with Sanofi became profitable for the first time in 2019, and we took important additional steps to strengthen Regeneron’s financial position. In the second quarter of 2020 we restructured our Antibody Collaboration with Sanofi to enhance profitability further and to simplify the commercial strategy for Praluent®(alirocumab). We will continue to collaborate with Sanofi on studying Kevzara for COVID-19, with Regeneron leading U.S.-based development and Sanofi leading development outside of the United States.
Meanwhile our research and development productivity continues, with notable progress in our growing immuno-oncology portfolio and diversification across the pipeline as a whole. We expanded our clinical program with Libtayo®(cemiplimab-rwlc), a PD-1 inhibitor, and moved multiple bispecific antibodies into the clinic, including the first in a whole new class of co-stimulatory bispecifics, which position us to become a leader in this emerging field. We continue to explore Dupixent in a variety of additional Type 2 inflammatory conditions, with late-stage trials underway in eosinophilic esophagitis, chronic obstructive pulmonary disease, prurigo nodularis, and chronic spontaneous urticaria, as well as earlier studies in grass and food allergies.
From a public health perspective, we made a breakthrough in the fight against the devastating Ebola outbreak in the Democratic Republic of the Congo (DRC) with REGN-EB3’s impressive reduction in mortality compared to the prior
 |
|
|
|
|
|
|
|
standard-of-care in the PALM clinical trial. We are applying the same technologies against the novel coronavirus and hope for similar success.
All of this important research and groundbreaking medicine is built on three decades of investment in ourVelociSuite®technologies. These proprietary end-to-end drug discovery and development tools allow us to quickly identify multiple antibody candidates against diseases – from Ebola to cancer to asthma to rare diseases, such as fibrodysplasia ossificans progressiva. Paired with our excellent clinical development and manufacturing capabilities, the possibilities are truly endless, and we feel that we are just at the beginning of what we can do using the power of science and technology.
Our commitment to continually advancing research through sophisticated and broadly applicable technology propelled our Regeneron Genetics Center®team to the major milestone of sequencing the exomes of over one million people as of February 2020. We are also applying our genetics and biology expertise to explore new modalities that are complementary to our world-class therapeutic antibodies. Key examples include our preclinical work in viral vector and gene therapy technologies, as well as ongoing collaborations with organizations who bring unique expertise in areas like gene silencing, gene editing, and CAR-Ts.
In 2020 and beyond, we will continue to reinvest a significant portion of our growing revenue into our R&D efforts as we believe our scientific innovation and talent are our greatest differentiators. As we look toward the future of Regeneron we have begun to evaluate ex-U.S. commercialization opportunities, starting with exercising our co-commercialization rights for Dupixent in certain countries outside the U.S.
2019 was a busy and successful year for Regeneron, and we believe 2020 will be even more impactful. We will innovate against COVID-19 and the other serious diseases that continue to impact lives, even during a time of pandemic. We have our sights firmly set on the future as we expand the types of ailments we can treat and number of people we can help. The world needs us and the power of science, more than ever.
Sincerely,
|
|
 |
 | |
| P. Roy Vagelos, | Leonard S. Schleifer, | George D. Yancopoulos, |
| M.D. | M.D., Ph.D. | M.D., Ph.D. |
|
|
|
|
|
| Co-Founder, President and | Co-Founder, President and |
| Chief Executive Officer | Chief Scientific |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
|
|

2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING


![]()
REGENERON PHARMACEUTICALS, INC.
777 Old Saw Mill River Road
Tarrytown, New York 10591-6707
| NOTICE OF ANNUAL MEETING OF SHAREHOLDERS |
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
The 20162020 Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. (the "Company"“Company”) will be held on Friday, June 10, 2016,12, 2020, commencing at 10:30 a.m., Eastern Time, virtually via the Internet and at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York, for the following purposes:
(1)to elect three Class I directors for a term of three years;(2)to ratify the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016; and(3)to act upon such other matters as may properly come before the meeting and any adjournment(s) or postponement(s) thereof.
| 1 | to elect four Class II directors for a three-year term and one Class III director for a one-year term; |
| 2 | to ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2020; |
| 3 | to approve the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan; |
| 4 | to cast an advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say on pay); and |
| 5 | to act upon such other matters as may properly come before the meeting and any adjournment(s) or postponement(s) thereof. |
The board of directors has fixed the close of business on April 14, 20162020 as the record date for determining shareholders entitled to notice of, and to vote at, the Annual Meeting and at any adjournment(s) or postponement(s) thereof.
Pursuant to the rules of the Securities and Exchange Commission (the “SEC”), we have elected to use the "Notice“Notice and Access"Access” method of providing our proxy materials over the Internet. Accordingly, we will mail, beginning on or about April 27, 2016,24, 2020, a Notice of Internet Availability of Proxy Materials to our shareholders of record and beneficial owners as of the record date (other than (i) those who previously elected to access thereceive proxy materials over the Internet,by e-mail, (ii) those who have previously asked to receive paper copies of the proxy materials, and (iii) shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan). As of the date of mailing of the Notice of Internet Availability of Proxy Materials, all shareholders and beneficial owners will have the ability to access all of the proxy materials on a website referenced in the Notice of Internet Availability of Proxy Materials.
The Notice of Internet Availability of Proxy Materials also contains a toll-free telephone number, an e-mail address, and a website where shareholders can request a paper or electronic copy of the proxy statement, our 20152019 annual report, and/or a form of proxy relating to the Annual Meeting. These materials are available free of charge. The Notice also contains information on how to access and vote the form of proxy.
Due to concerns regarding the coronavirus outbreak (“COVID-19”) and to assist in protecting the health and well-being of our shareholders, directors, and employees, shareholders will be able to attend the meeting and participate electronically as part of the virtual meeting format of the Annual Meeting. This additional means of attending allows shareholders the opportunity to vote their shares on the date of the Annual Meeting even if they are not able or do not wish to attend the meeting in person. In addition, the meeting’s virtual attendance option provides shareholders the ability to participate and ask questions during the meeting. As required by New York law, shareholders have the option to attend the Annual Meeting in person. If the legal requirement to include an in-person option is waived by relevant governmental action, we may opt to hold the Annual Meeting as a virtual-only meeting. We may also change the venue for the in-person meeting option if required by the circumstances. In any such case, we would notify our shareholders in advance on our website and by issuing a press release and filing it as additional proxy material with the SEC. We strongly encourage shareholders to attend virtually in light of COVID-19 and public health concerns and to visit our website athttp://newsroom.regeneron.comfor the most up-to-date information on the Annual Meeting, any procedures and limitations concerning in-person attendees, and information regarding any government-imposed limits on public gatherings applicable to the Annual Meeting that may be in effect at that time.
As Authorized by the Board of Directors,

Joseph J. LaRosa
Executive Vice President, General Counsel and Secretary
April 24, 2020
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |

 |

TABLE OF CONTENTS
 | 1 |
April 26, 2016
REGENERON PHARMACEUTICALS, INC.
![]()
i
Table of Contents
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / i / i |
TABLE OF CONTENTS (CONT.)
Note Regarding Forward-Looking Statements and Non-GAAP Financial Measures
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND NON-GAAP FINANCIAL MEASURES: See Appendix A for important information regarding forward-looking statements and financial measures not calculated in accordance with U.S. Generally Accepted Accounting Principles contained in this proxy statement.
ii /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
ii
Table of Contents

![]()
Proxy Summary
The summary below highlights information that is described in more detail elsewhere in this proxy statement. This summary does not contain all of the information you should consider, and we urge you to read the entire proxy statement carefully before voting.
General Information (see "General Information about the Meeting" on page 6 for more information)PROXY DASHBOARD
GENERAL INFORMATION
| Meeting Date: | ||||||
| Time: | Record Date: | |||||
| JUNE 12, 2020 | 10:30 A.M., ET | ONLINE www.virtualshareholdermeeting.com/REGN2020 | APRIL 14, 2020 | |||
| OR IN PERSON | ||||||
| Westchester Marriott Hotel | ||||||
| 670 White Plains Road | ||||||
| Tarrytown, New York 10591 | ||||||
Meeting Agenda
MEETING AGENDA
| Matter | Board Vote Recommendation | |||
| 1 | Election of |  | ||
| 2 | Ratification of the appointment of PricewaterhouseCoopers LLP as the | FOR | ||
| 3 | Approval of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | FOR | ||
| 4 | Advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say on pay) | FOR |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 1 / 1 |
USERS’ GUIDE / PROXY HIGHLIGHTS
WE SEEK YOUR INPUT ON TWO COMPENSATION-RELATED MATTERS
Our mission of long-term commitment to science and innovation has shaped our compensation program, which is designed to sustain our business model and drive our product pipeline. We seek your vote on two compensation-related proposals:
| 1 | Approval of the second amendment and restatement of our long-term incentive plan—see “Proposal No. 3: Second Amendment and Restatement of Long-Term Incentive Plan”; and |
| A non-binding proposal to approve the compensation of our Named Executive Officers—see “Proposal No. 4: Advisory Vote on Compensation of Named Executive Officers (Say on Pay).” |
As discussed under “Compensation-Related Matters—Introduction,” our compensation model has been instrumental in creating a culture of loyal and motivated employees with an entrepreneurial spirit who are dedicated to the Company’s mission to use the power of science to invent medicines for people with serious diseases. As you consider voting on these proposals, please keep in mind the following:
| • | Our equity compensation program supports all of our employees, not just our Named Executive Officers, with approximately 90% of recent annual equity grants awarded to employees other than our Named Executive Officers. |
| • | We granted performance-based restricted stock units to our CEO and CSO as a component of their 2019 annual equity awards. This and other carefully calibrated changes to our compensation program were based on investor feedback. |
| • | Our 2019 burn rate was at the lowest level in the last seven years due to specific steps we took to manage dilution from equity compensation. |
| • | We are asking our shareholders to approve the same number of shares for equity grants that we requested previously and are committed to ensuring that shares available under our long-term incentive plan will be sufficient for at least three years. |
| • | Our compensation model underpins our strategy of creating and advancing a high-quality, internally developed product pipeline, which delivered six important medicines and eight additional key indications for these products in the past decade. |
Proposal No. 1 –
Your support on each of these proposals will help us continue to develop the product pipeline that drives our performance and to harness the power of science for the benefit of people with serious diseases.
WE SEEK YOUR INPUT ON OUR BOARD
The composition of our board of directors reflects our core principle of “science first”: over half of our directors are members of the National Academy of Sciences, and our board members include two Nobel laureates and holders of many scientific awards. By having our board of directors heavily populated with top scientific talent, we signal to our shareholders and employees our seriousness about the Company’s dedication to science and its core competencies and primary value driver. Our Director Nominees (see "Proposalboard also includes individuals with experience building shareholder value through all stages of corporate development, as well as governance, financial, and policy expertise. Five of our board’s current 12 members are diverse by gender, race, or national origin.
Please refer to “Proposal No. 1: Election of Directors" on page 10Directors” for more information)
The following individuals have been nominated for election at the 2016 Annual Meeting:
| Director Class | Name | Age* | Director Since | Occupation | Independent | Committee Memberships | ||||||
| Class I | Michael S. Brown, M.D. | 75 | 1989 | Distinguished Chair in Biomedical Sciences, Regental Professor of Molecular Genetics, and Director of the Jonsson Center for Molecular Genetics, University of Texas Southwestern Medical Center at Dallas | ü | Technology Committee (Chairman) Corporate Governance and Compliance Committee | ||||||
| Class I | Leonard S. Schleifer, M.D., Ph.D. | 63 | 1988 | President and Chief Executive Officer of Regeneron Pharmaceuticals, Inc. | | Technology Committee (Ex Officio Member) | ||||||
| Class I | George D. Yancopoulos, M.D., Ph.D. | 56 | 2001 | President, Regeneron Laboratories and Chief Scientific Officer of Regeneron Pharmaceuticals, Inc. | | Technology Committee (Ex Officio Member) | ||||||
| | | | | | | | | | | | | |
*As of April 14, 2016.
1
Proxy Summary
Each director nominee is a current director and attended at least 75% of the aggregate of all 2015 meetings of the board of directors and each committee on which he served.
Corporate Governance (see "Corporate Governance" on page 15 for more information)additional information.
Regeneron is committedWE SEEK RATIFICATION OF OUR AUDITORS
We pay close attention to good corporate governance, whichthe requirements applicable to us as a publicly traded company, including those relating to the audit of Regeneron’s financial statements by our independent registered public accounting firm, PricewaterhouseCoopers LLP. In this proxy statement, we believe promotesare asking you to ratify the long-term interests of shareholders, strengthens the accountability of the board of directors and management, and helps build trust in the Company. The following chart summarizes key information regarding our corporate governance.
*As of April 14, 2016.
Proposal No. 2 – Ratificationappointment of PricewaterhouseCoopers LLP (see "Proposal as our independent registered public accounting firm for the fiscal year ending December 31, 2020.
Please refer to “Proposal No. 2: Ratification of Appointment of Independent Registered Public Accounting Firm" on page 33Firm” for more information)additional information.
We ask that our shareholders ratify the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for 2016. Below is a summary of fees related to services provided to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 2015 and 2014.
| | 2015 | | 2014 | ||||
Audit Fees | $ | 1,721,000 | $ | 1,567,493 | |||
Audit-Related Fees | | 2,007 | | – | |||
All Other Fees | | 4,637 | | 4,812 | |||
| | | | | | | | |
Total Fees | $ | 1,727,644 | $ | 1,572,305 |
2015 Performance Overview (see "Executive Compensation – Compensation Discussion and Analysis – Section 1 – Summary – 2014 Performance Overview" on page 35 for more information)
2015 was another extraordinary year for Regeneron. Our key accomplishments in 2015 included:
•47% growth in EYLEA® (aflibercept) Injection global net product sales as compared to 2014;
•46% growth in our total revenues as compared to 2014;•19% growth in non-GAAP net income as compared to 2014 (non-GAAP net income is not a measure calculated in accordance with U.S. Generally Accepted Accounting Principles; see Appendix A for a definition of non-GAAP net income and a reconciliation of non-GAAP net income to net income);•advances in our EYLEA® franchise, including regulatory approval of EYLEA® for the treatment of visual impairment due to macular edema secondary to retinal vein occlusion and the treatment of visual impairment secondary to myopic choroidal neovascularization in the European Union; regulatory approval of EYLEA® for the treatment of diabetic retinopathy in patients with diabetic macular edema in the United States; and regulatory approval of EYLEA® for the treatment of retinal vein occlusion in Japan;•regulatory approval and launch of Praluent® (alirocumab) Injection, the first drug approved by the U.S. Food and Drug Administration ("FDA") in a new class of drugs that lower LDL ("bad") cholesterol;•positive Phase 3 data for sarilumab from three Phase 3 studies in patients with rheumatoid arthritis (SARIL-RA-TARGET, SARIL-RA-EASY, and SARIL-RA-ASCERTAIN) and submission of a Biologics License Application for sarilumab with the FDA;
2
Proxy Summary
•positive pivotal Phase 2b data for dupilumab in asthma and completion of enrollment of the dupilumab atopic dermatitis Phase 3 studies;•new collaboration agreement relating to fasinumab with Mitsubishi Tanabe Pharma Corporation for Japan, Korea, and nine other Asian countries, excluding China;•initiation of Phase 3 clinical study of REGN2222 for Respiratory Syncytial Virus;•continued growth of our clinical development pipeline, as evidenced by the submission of one Investigational New Drug Application with the FDA in 2015 and 13 product candidates (consisting of one Trap-based and 12 fully-human monoclonal antibody product candidates based on the Company'sVelocImmune®technology) in clinical development as of December 31, 2015;•new global strategic collaboration with Sanofi to discover, develop, and commercialize antibody-based cancer treatments in the field of immuno-oncology; and•further important steps to support our current and future growth, including adding two new buildings in the Tarrytown campus providing nearly 300,000 square feet of additional laboratory and office space; significant progress with the construction of a new manufacturing facility in Limerick, Ireland; and increasing headcount on a year-over-year basis by approximately 47% as of December 31, 2015.
Our strong performance is reflected in the appreciation of our stock price, which increased 32%, 217%, and 1554% over the one-, three-, and five-year periods ended December 31, 2015, respectively. This shareholder return places our common stock performance in the 85th, 90th, and 99th percentile, respectively, of all NASDAQ-listed companies with a market capitalization greater than $5 billion in those periods.
Executive Compensation (see "Executive Compensation" on page 35 for more information)
We believe that the leadership of the current executive team has been instrumental to our success in 2015 and prior years, and that an executive compensation program that attracts, motivates, and helps retain key executives, including the Named Officers, is critical to our long-term success.
The main objectives of our executive compensation program are to pay for performance; closely align the interests of shareholders and management; strike a balance between short- and long-term perspectives and support our long-term growth prospects; and attract and retain highly skilled and talented executives in a competitive marketplace.
These objectives were reflected in our 2015 compensation decisions in a number of ways, including the following:
•We believe in performance-based compensation and long-term incentives.In 2015, we continued to rely primarily on performance-based compensation, both for our short-term (cash bonus) and long-term incentives (stock option awards). This emphasis on performance-based compensation (particularly long-term incentives in the form of stock options) has been a consistent part of our philosophy since Regeneron's inception, including prior to the significant appreciation in Regeneron's stock price that began in early 2011.•We believe that time-based stock options are inherently performance based, as they provide value to employees only if there is future stock price appreciation and do not provide any value to employees if the stock price declines below the exercise price.As illustrated by the charts in "Executive Compensation – Compensation Discussion and Analysis – Section 2 – Analysis of 2015 Executive Compensation Based on Compensation Objectives," this emphasis on stock options has resulted in close alignment of our Chief Executive Officer's compensation in 2015 and over the last five years with the performance of our common stock over those periods:oBoth in 2015 and over the five-year period ended December 31, 2015, the year-over-year increases in our Chief Executive Officer's compensation were principally attributable to the significant appreciation in our stock price, which increased the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model for valuing stock options.oOver the same periods, the Black-Scholes grant date fair value of stock option grants to our Chief Executive Officer increased less than the appreciation of our stock price, in part because the absolute number of stock options granted to our Chief Executive Officer decreased in the last three years. The number of shares underlying the annual stock option award to our Chief Executive Officer in 2015 was approximately 39% lower than in 2012, while the stock price appreciated 217% over the same period. As a result, the appreciation in the reported value of our Chief Executive Officer's pay was significantly below the appreciation of our stock price, both cumulatively over the five-year period and on a year-over-year basis. This means that the value of our long-term shareholders' investment in Regeneron grew more rapidly than our CEO's pay over those periods.oTo further illustrate this point, over the last five years, our Chief Executive Officer's total direct compensation, as a percentage of Regeneron's capitalization in the year in which the compensation was awarded, decreased from 0.20% to 0.08%.
3
Proxy Summary
oAs a result of our emphasis on performance-based compensation, on a relative basis when compared to our Peer Group, the total direct compensation of our Chief Executive Officer over the last three years was also closely aligned with the performance of our common stock even when taking into account the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model.
•We believe in year-over-year consistency in making compensation decisions and in striking a balance between the dilutive impact of equity grants and the competitiveness of our compensation program.In our compensation decisions, we focus on the number of shares underlying equity awards relative to the number of basic shares of common stock outstanding, rather than the grant date fair value of the award (as determined according to the Black-Scholes model). We believe this ownership- and dilution-based approach to awarding stock options provides a better measure of the amount of potential increases in shareholder value that would be shared by the awards and allows us to evaluate such grants on a consistent basis as compared to other companies and regardless of fluctuations in the price of Regeneron's or other companies' common stock. Further, focusing on the number of shares and the incremental sharing rate of potential future upside (rather than targeting a specific Black-Scholes grant date fair value) avoids rewarding officers with larger grant sizes following a decline in our stock price.oAs a percentage of the total basic shares outstanding, the 2015 stock option award to our Chief Executive Officer was significantly below the 75thpercentile of the companies included in the 2015 Radford Global Life Sciences Survey and only slightly above the 50thpercentile (at 0.166% compared to 0.290% and 0.154%, respectively). In addition, this award was below the 50thpercentile of our Biotech R&D Peers (which was 0.183%).oIn 2015, the Compensation Committee reduced the number of shares underlying the annual stock option awards to the Named Officers by 15% compared to 2014 (other than
Mr. Terifay's award, which remained at the 2014 level due to his promotion to Executive Vice President, Commercial). This decrease constituted the third consecutive double-digit percentage decrease in the annual grant of stock options to our Named Officers, in each case following outstanding TSR performance. In reducing the size of 2015 annual stock option awards to executives, the Compensation Committee sought to reduce the potential dilutive impact of new equity awards without adversely affecting the competitiveness of our executive compensation program, which has successfully motivated our senior management team to deliver high operating performance and shareholder value.oWe continued to pay close attention to our burn rate. Despite the expansive growth of our employee base, which increased by 121% between 2012 and 2015 (from 1,950 full-time employees to 4,303 full-time employees), our burn rate decreased from 5.4% to 4.4% over the same period, and we maintained a three-year burn rate average of 4.1% in 2015. We achieved this reduction through implementing three consecutive double-digit percentage decreases in the number of shares underlying annual stock option awards, without eliminating the broad-based nature of our equity compensation program.oWe believe our approach to equity compensation has helped us to successfully grow and manage employee attrition, as evidenced by our 2015 employee turnover of approximately 6%, which compares favorably to the average employee turnover of approximately 18% for the life sciences sector based on the Fourth Quarter 2015 Radford Global Life Sciences Trends Report.
Our Compensation Policies and Practices
We have compensation policies and practices designed to enhance governance of our executive compensation program and to further our compensation objectives. These policies and practices include:
2 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
4
Proxy Summary
5
USERS’ GUIDE/ Proxy SummaryGENERAL INFORMATION ABOUT THE MEETING
REGENERON PHARMACEUTICALS, INC.777 Old Saw Mill River RoadTarrytown, New York 10591-6707
April 26, 2016
![]()
General Information about the Meeting
GENERAL INFORMATION ABOUT THE MEETING
ANNUAL MEETING INFORMATION
Where and when willWhen is the 2016 Annual Meeting be held?Meeting?
The 2016
June 12, 2020
What time is the Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. ("Regeneron," "Company," "we," "us," and "our") is scheduled for June 10, 2016, commencing at Meeting?
10:30 a.m., Eastern Time,ET
Where is the Annual Meeting?
Virtually via the Internet atwww. virtualshareholdermeeting.com/REGN2020and at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York 10591. If you are planningthe legal requirement to include an in-person option is waived by relevant governmental action, we may opt to hold the Annual Meeting as a virtual-only meeting. We may also change the venue for the in-person meeting option if required by the circumstances. In any such case, we would notify our shareholders in advance on our website and by issuing a press release and filing it as additional proxy material with the SEC.
What form of identification do I need to be admitted to the meeting?
Via the Internet.Instructions on how to attend and participate via the Internet, including how to demonstrate proof of stock ownership, are posted atwww.virtualshareholdermeeting.com/REGN2020.To vote during the meeting, you will need the 16-digit control number included on the Notice of Internet Availability of Proxy Materials (the “Notice”) or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received.
In person.You will be asked to present valid, government-issued photo identification, such as a driver’s license.
Where can I find directions to the in-person Annual Meeting?
Directions to this location are available on our website athttp://newsroom.regeneron.comnewsroom.regeneron.com.In-person attendance will be subject to any government-imposed limitations on public gatherings then in effect. As noted above, under certain circumstances we may opt to hold
the Annual Meeting as a virtual-only meeting. Please refer to our website for the most up-to-date information.
Can I vote at the Annual Meeting?
Only shareholders of record at the close of business on the record date, April 14, 2020, are entitled to vote at the Annual Meeting. As of April 14, 2020, 110,673,311 shares of the Company’s common stock, par value $0.001 per share (“common stock”), and 1,848,970 shares of Class A stock, par value $0.001 per share (“Class A stock”), were issued and outstanding. The common stock and the Class A stock vote together on all matters as a single class, with the common stock being entitled to one vote per share and the Class A stock being entitled to ten votes per share.
What is on the agenda for the meeting?
| 1 | Election of four Class II directors for a three-year term and one Class III director for a one-year term |
| 2 | Ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2020 |
| 3 | Approval of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan |
| 4 | Advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say on pay) |
Can I ask a question at the Annual Meeting?
Via the Internet.Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be able to submit questions during the meeting.
In person.Attendees of the meeting will be given an opportunity to ask questions during a designated question-and-answer period.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 3 / 3 |
USERS’ GUIDE/.GENERAL INFORMATION ABOUT THE MEETING
VOTING INFORMATION
Why did youI receive a notice in the mail regarding the Internet availability of proxy materials instead of a paper copy of the proxy materials?
The "Notice“Notice and Access"Access” rules of the United States Securities and Exchange Commission (the "SEC"“SEC”) permit us to furnish proxy materials, including this proxy statement, and our Annual Report on Form 10-K for the fiscal year ended December 31, 20152019 filed with the SEC on February 11, 20167, 2020 (the "2015“2019 Annual Report"Report”), to our shareholders by providing access to such documents on the Internet instead of mailing printed copies. Most shareholders received athe Notice of Internet Availability of Proxy Materials (the "Notice") and will not receive printed copies of the proxy materials unless they request them. This method reduces the environmental impact of the Annual Meeting. The Notice will be mailed beginning on or about April 27, 2016.24, 2020. The Notice includes instructions on how you may access and review all of our proxy materials and the 2019 Annual Report via the Internet. The Notice also includes instructions on how you may vote your shares. If you would like to receive a paper or electronic copy of our proxy materials, you should follow the instructions in the Notice for requesting such materials. Any request to receive proxy materials by mail or e-mail will remain in effect until you revoke it.
Why didn't you receive a notice in the mail about the Internet availability of the proxy materials?
Shareholders who previously elected to access the proxy materials over the Internet will not receive a notice in the mail about the Internet availability of the proxy materials. Instead,
these shareholders should have received an e-mail with links to the proxy materials and the proxy voting website. In addition, shareholders who have previously asked to receive paper copies of the proxy materials and shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan will receive paper copies of the proxy materials.
Can youI vote yourmy shares by filling out and returning the Notice?
No. The Notice identifies the items to be voted on at the Annual Meeting, but you cannot vote by marking the Notice and returning it. The Notice provides instructions on how to vote by Internet, by requesting and returning a paper proxy card, or by submitting a ballot in personvoting at the meeting.
Why did we send youI receive the Notice?
We sent you the Notice regarding this proxy statement because Regeneron'sRegeneron’s board of directors is asking (technically called soliciting) holders of the Company's common stock par value $0.001 per share ("common stock"), and Class A stock par value $0.001 per share ("Class A stock"), to provide proxies to be voted at our 20162020 Annual Meeting of Shareholders or at any adjournment(s) or postponement(s) of the meeting.
Who is entitled to vote at the Annual Meeting?
Only shareholders of record at the close of business on the record date, April 14, 2016, are entitled to vote at the Annual Meeting shares of common stock and/or Class A stock held of record on that date. As of April 14, 2016, 103,165,457 shares of common stock and 1,913,136 shares of Class A stock were issued and outstanding. The common stock and the Class A stock vote together on all matters as a single class, with the common stock being entitled to one vote per share and the Class A stock being entitled to ten votes per share.
6
General Information about the Meeting
What are you being asked to vote on?
We are asking you to vote on:
•election of three Class I directors for a term of three years (Proposal No. 1); and•ratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016 (Proposal No. 2).
What are the board's recommendations?
The board of directors recommends that you vote:
•FORelection of each of the three nominated Class I directors (Proposal No. 1); and•FORratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for 2016 (Proposal No. 2).
How can you vote?
In person. If you are a shareholder of record, you may vote in person at the Annual Meeting. The Company will give you a ballot when you arrive. If you are a beneficial owner of shares held in the name of your bank, broker, or other nominee, or in "street name," to vote in person at the Annual Meeting you must obtain from your nominee and bring to the meeting a "legal proxy" authorizing you to vote such shares held as of the record date. We recommend you vote by proxy even if you plan to attend the meeting. So long as you meet the applicable requirements, you can always change your vote at the meeting. Instructions on voting by proxy are included below.
Via the Internet. You may vote by proxy via the Internet by visitingwww.proxyvote.com. You will need the 12 digit control number included on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received. You may vote via the Internet through 11:59 p.m., Eastern Time, on June 9, 2016.
Via telephone. If you received printed copies of the proxy materials, you may vote by proxy via telephone by calling the toll free number found on the proxy card or the voting instruction form. You will need the 12 digit control number included on the proxy card or voting instruction form. You may vote via telephone through 11:59 p.m., Eastern Time, on June 9, 2016.
By mail. If you received printed copies of the proxy materials, you may vote by proxy by completing the proxy card or voting instruction form and returning it in the envelope provided.
How are proxies voted?
If you vote by proxy in time for it to be voted at the Annual Meeting, one of the individuals named as your proxy will vote your shares as you have directed. If you submit a proxy, but no indication is given as to how to vote your shares as to a proposal, your shares will be voted in the manner recommended by the board of directors. The board of directors knows of no matter, other than those indicated above under "What are you being asked to vote on?"“What is on the agenda at the meeting?”, to be presented at the Annual Meeting. If any other matter properly comes before the Annual Meeting, the persons named and designated as proxies will vote your shares in their discretion.
Why didn’t I receive a notice in the mail about the Internet availability of the proxy materials?
Shareholders who previously elected to receive proxy materials by e-mail will not receive a notice in the mail about the Internet availability of the proxy materials. Instead, these shareholders should have received an e-mail with links to the proxy materials and the proxy voting website. Shareholders who have previously asked to receive paper copies of the proxy materials and shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan will receive paper copies of the proxy materials.
What constitutes a quorum?
The presence at the Annual Meeting, in person or by proxy, of the holders as of the record date of shares of common stock and Class A stock having a majority of the voting power of all shares of common stock and Class A stock outstanding on the record date will constitute a quorum for the transaction of business at the Annual Meeting. Shares held as of the record date by holders who are present or represented by proxy at the Annual Meeting but who have abstained from voting or have not voted with respect to some or all of such shares on any proposal to be voted on at the Annual Meeting will be counted as present for purposes of establishing a quorum.
7
General Information about the Meeting
What vote is required to approve each proposal?
The following table summarizes the voting requirements applicable to the proposals to be voted on at the Annual Meeting:
4 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
*USERS’ GUIDE/
How can I vote my shares without attending the Annual Meeting.
+Only relevantMeeting?We recommend that shareholders vote by proxy even if
you arethey plan to attend thebeneficial owner of shares heldAnnual Meeting via the Internet or in"street name."person. If you are a shareholder of record,andthere are three ways to vote by proxy:Via the Internet.You may vote by proxy via the Internet by visitingwww.proxyvote.com.You will need the 16-digit control number included on the Notice or, if you
do not cast your vote, no votes will be cast on your behalf on anyreceived a paper copy of theitemsproxy materials, the proxy card or voting instruction form you received. You may vote via the Internet through 11:59 p.m., Eastern Time, on June 11, 2020.Via telephone.You may vote by proxy via telephone by calling the toll-free number found on the proxy card or the voting instruction form. You will need the 16-digit control number included on the proxy card or voting instruction form. You may vote via telephone through 11:59 p.m., Eastern Time, on June 11, 2020.
By mail.If you received printed copies of
businessthe proxy materials, you may vote by proxy by completing the proxy card or voting instruction form and returning it in the envelope provided.How can I attend and vote at the Annual
Meeting.
Via the Internet.You may vote via the Internet atwww.virtualshareholdermeeting.com/REGN2020where you will be able to vote during the meeting. Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be able to vote during the meeting.
In person.If you are a shareholder of record, you may vote in person at the Annual Meeting. The Company will give you a ballot when you arrive. If you are a beneficial owner of shares held in the name of your bank, broker, or other nominee, or in “street name,” to vote in person at the Annual Meeting you must obtain from your nominee and bring to the meeting a “legal proxy” authorizing you to vote such shares held as of the record date. We recommend you vote by proxy even if you plan to attend the meeting. So long as you meet the applicable requirements, you can always change your vote at the meeting. Instructions on voting by proxy are included below. As noted above, under certain circumstances we may opt to hold the Annual Meeting as a virtual-only meeting. Please refer to our website for the most up-to-date information.
What if during the Annual Meeting I have technical difficulties or trouble accessing the virtual meeting website?
We will have technicians ready to assist you with any technical difficulties you may have accessing the virtual meeting website. If you encounter any difficulties accessing the virtual meeting website during the meeting time, please call the technical support number that will be posted atwww.virtualshareholdermeeting.com/ REGN2020.
If I am a Regeneron employee or former employee, how do youI vote shares in the Company Stock Fund in yourmy 401(k) account?account or in the Regeneron Ireland Share Participation Plan?
If you participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan, you may provide voting instructions to Fidelity Management Trust Company, the plan'splan’s trustee, (1) through the Internet atwww.proxyvote.comby 11:59 p.m., Eastern Time, on June 7, 2016,9, 2020, (2) by calling 1-800-690-6903 by 11:59 p.m., Eastern Time, on June 7, 2016,9, 2020, or (3) by returning your completed proxy card by mail. The trustee will vote your shares in accordance with your instructions. If you do not provide timely voting instructions to the trustee, the trustee will vote your shares in the same proportion as the shares for which the trustee receives voting instructions from other participants in the plan.
If you participate and hold shares of common stock in the Regeneron Ireland Share Participation Plan, you may provide voting instructions to Mercer Ireland Limited, who administers the Plan on behalf of Irish Pensions Trust Limited, the trustees of the Plan. You will receive a voting instruction form by mail sent directly to your home address, which you should complete, sign, and return to Mercer by mail using the enclosed pre-paid envelope or as an e-mail attachment in accordance with the instructions provided by Mercer.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 5 / 5 |
USERS’ GUIDE/GENERAL INFORMATION ABOUT THE MEETING
Can youI change yourmy vote or revoke yourmy proxy?
Yes. You may change your vote or revoke your proxy at any time before the proxy is exercised. If you votedexercised by proxyvoting again electronically through the Internet or by telephone, as described above,by mailing a new proxy card or voting instruction form, or by attending the Annual Meeting and voting. If you are a record holder, you may simply vote again at a later date using the same procedures, in which case the later submitted proxy will be recorded and the earlier vote revoked. If you submittedalso revoke your proxy by mail, you must (i) filefiling with the
Secretary of the Company, at or before the taking of the vote at the Annual Meeting, a written notice of revocation bearing a later date than the proxy you previously submitted or (ii) duly execute a later dated proxy relating to the same shares and deliver it to the Secretary of the Company or other designee before the taking of the vote at the Annual Meeting.submitted. Attendance at the Annual Meeting will not have the effect of revoking a proxy unless you are a record holder and give written notice of revocation to the Secretary of the Company before the proxy is exercised or you vote by written ballot at the Annual Meeting. If you hold your shares through a broker, bank, or other nominee in "street“street name,"” you will need to contact them or follow the instructions in the voting instruction form used by the firm that holds your shares to revoke your proxy. Only your latest dated proxy we receive at or prior to the Annual Meeting will be counted.
Who solicits proxies and bears the cost of solicitation?
Solicitation of proxies may be made by mail, in person, or by telephone by officers, directors, and other employees of the Company and by employees of the Company'sCompany’s transfer agent, American Stock Transfer & Trust Company, LLC ("AST"(“AST”), and employees of Broadridge Financial Solutions, Inc. ("Broadridge"(“Broadridge”). We will reimburse AST, Broadridge, and our banks, brokers, and other custodians, nominees, and fiduciaries for their respective reasonable costs in the preparation and mailing of proxy materials to shareholders. In addition, we
8
General Information about the Meeting
have engaged Innisfree M&A Incorporated to assist in the solicitation of proxies and provide related advice and informational support for a servicesservice fee of $25,000 and the reimbursement of customary disbursements that are not expected to exceed $25,000 in the aggregate.and expenses. We will bear all costs of the solicitationof proxies.
What are the board’s recommendations?
The board of directors recommends that you vote:
 | FORelection of each of the four nominated Class II directors and the one nominated Class III director (Proposal No. 1); |
 | FORratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for 2020 (Proposal No. 2); |
 | FORapproval of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (Proposal No. 3); and |
 | FORapproval of the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say on pay) (Proposal No. 4). |
6 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
USERS’ GUIDE/GENERAL INFORMATION ABOUT THE MEETING
What vote is required to approve each proposal?
The following table summarizes the voting requirements applicable to the proposals to be voted on at the Annual Meeting:
| Proposal | Vote Required | Effect of Abstentions* | Broker Discretionary Voting Allowed?+ | |
| 1 | Election of Directors | Majority of the votes cast. In accordance with our director resignation policy, an incumbent director who fails to receive the required number of votes in an uncontested election will be required to tender his or her resignation to the Chairman of the board of directors for consideration by the Corporate Governance and Compliance Committee. | No effect — not considered votes cast on this proposal | No — brokers without voting instructions will not be able to vote on this proposal |
| 2 | Ratification of the Appointment of PricewaterhouseCoopers LLP | Majority of the votes cast | No effect — not considered votes cast on this proposal | Yes — brokers without voting instructions will have discretionary authority to vote |
| 3 | Approval of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | Majority of the votes cast | No effect — not considered votes cast on this proposal | No — brokers without voting instructions will not be able to vote on this proposal |
| 4 | Say on Pay | Non-binding, advisory proposal. We will consider the matter approved if it receives the affirmative vote of a majority of the votes cast | No effect — not considered votes cast on this proposal | No — brokers without voting instructions will not be able to vote on this proposal |
| * | As noted above, abstentions will be counted as present for purposes of establishing a quorum at the Annual Meeting. |
| + | Only relevant if you are the beneficial owner of shares held in “street name.” If you are a shareholder of record and you do not cast your vote, no votes will be cast on your behalf on any of the items of business at the Annual Meeting. |
If any other matter is properly brought before the Annual Meeting, such matter also will be determined by the affirmative vote of a majority of the votes cast at the Annual Meeting.
Please note that cameras, other photographic equipment, or audio or video recording devices will not be permitted atto be used by any in-person attendees of the Annual Meeting.
9
General Information about the Meeting
![]()
Proposal No. 1: Election of Directors
Pursuant
INFORMATION ABOUT REGENERON
If you would like to learn more about Regeneron, please visit our website at www.regeneron.com. The topics discussed on our website include:
| • | Working at Regeneron | • | Our Graduate Internship Program | |
| • | Our Science Research Mentorship Program | • | Our Post-doctoral Training Program | |
| • | The Regeneron Science Talent Search | • | Regeneron employee volunteer programs | |
| • | The Regeneron International Science and Engineering Fair | • | Our patient support programs | |
| • | The Regeneron DNA Learning Center | • | Our environmental sustainability efforts | |
| • | STEM Teaching Fellowship | • | Our commitment to global transparency |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 7 / 7 |

From Left:N. Anthony Coles, M.D. / Arthur F. Ryan / Michael S. Brown, M.D. / George L. Sing / Bonnie L. Bassler, Ph.D. / Leonard S. Schleifer, M.D., Ph.D. / P. Roy Vagelos, M.D. / George D. Yancopoulos, M.D., Ph.D. / Christine A. Poon / Joseph L. Goldstein, M.D. / Huda Y. Zoghbi, M.D. / Marc Tessier-Lavigne, Ph.D.

As the Company'sfirst substantive order of business at the 2020 Annual Meeting, you have an opportunity to vote on five members of our board of directors. This is the right starting point not only because the board oversees Regeneron, but because understanding the Regeneron board leads to a better understanding of the Company and its business model.
As our President and CEO has observed, “Our dream when we started Regeneron was to build a company where the scientists would be the heroes.” The composition of Regeneron’s board reflects this founding principle: over half of our directors are members of the National Academy of Sciences, and our board members include two Nobel laureates and holders of many scientific awards. In addition, the board includes individuals with experience building shareholder value through all stages of corporate development. Various members bring substantial governance experience gained from service on other boards and others bring financial, policy, and management expertise. Five of our board’s current 12 members are diverse by gender, race, or national origin.
The five members of our board of directors nominated for reelection at the 2020 Annual Meeting are N. Anthony Coles, M.D., Joseph L. Goldstein, M.D., Christine A. Poon, P. Roy Vagelos, M.D., and Huda Y. Zoghbi, M.D., all of whom currently are Class II directors. Consistent with Article VI of the Company’s Certificate of Incorporation and Section 704 of the New York Business Corporation Law, the board of directors is divided intohas determined that it would be in the best interests of the Company and its shareholders to apportion the directorships equally among the three classes denominated Class I, Class II, and Class III, with members of the board so that each class holding office for staggered three-year terms. There are currently three members in Class I and Class II andconsists of four members in Class III. The respective terms ofdirectors. To effect this equal apportionment, the directors expire (in all cases, subject to the election and qualification of their successors and to their earlier death, resignation, or removal) as follows:
•The terms of the Class I Directors expire at the 2016 Annual Meeting;•The terms of the Class II Directors expire at the 2017 Annual Meeting; and•The terms of the Class III Directors expire at the 2018 Annual Meeting.
The board of directors, upon the recommendation of the Corporate Governance and Compliance Committee, has nominated for election at the 20162020 Annual Meeting Michael S.
Brown,Joseph L. Goldstein, M.D., Leonard S. Schleifer,Christine A. Poon, P. Roy Vagelos, M.D., Ph.D., and George D. Yancopoulos,Huda Y. Zoghbi, M.D., Ph.D. as Class III Directors for a three-year term expiring at the 20192023 Annual Meeting.
Biographical information is given below,Meeting and N. Anthony Coles, M.D. as of April 14, 2016, for each nominee fora Class IIII Director and for each of the other directors whose term of office will continue after the 2016 Annual Meeting. All the nominees are presently directors and were previously elected by the shareholders. None of the corporations or other organizations referred to below with which a director has been or is currently employed or otherwise associated is a parent, subsidiary, or affiliate of the Company.
The board of directors unanimously recommends a vote FOR the election of Michael S. Brown, M.D., Leonard S. Schleifer, M.D., Ph.D., and George D. Yancopoulos, M.D., Ph.D. as Class I Directors for a three-yearone-year term expiring at the 20192021 Annual Meeting.
The table below summarizes key qualifications, skills, or attributes most relevant to the decision to nominate the director to serve on the board of directors. A mark indicates a specific area of focus or expertise on which the board of directors relies most. The lack of a mark does not mean the director does not possess that qualification or skill. Each director biography below describes these qualifications and relevant experience in more detail. We believe the table below demonstrates the breadth and diversity of the collective experience, expertise, and skills of our board of directors.
Experience, | Ph.D. | Michael S. Brown, M.D. | N. Anthony Coles, M.D. | Joseph L. Goldstein, M.D. | Christine A. Poon | Arthur F. Ryan | Leonard S. Schleifer, M.D., Ph.D. | George L. Sing | Marc Tessier- Lavigne, Ph.D. | P. Roy Vagelos, M.D. | George D. Yancopoulos, M.D., Ph.D. | ||||||||||||||
| Zoghbi, M.D. | ||||||||||||||||||||||||
| Industry Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Executive/Leadership Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Science/Biotech Background | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Research/Academic Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Business Strategy/ Operations Experience | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Financial Expertise | ● | ● | ● | ● | ● | ● | |||||||||||||||||||
| Public Company CEO Experience | |||||||||||||||||||||||||
National Academy of Sciences Membership | |||||||||||||||||||||||||
10
Proposal No. 1: Election of Directors
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
|
BOARD OF DIRECTORS / MEET THE BOARD
NOMINEES FOR CLASS II DIRECTORS
FOR ELECTION AT THE 2020 ANNUAL MEETING FOR A TERM EXPIRING AT THE 2023 ANNUAL MEETING1
JOSEPH L. GOLDSTEIN, M.D.

Director since:1991
Age:79
Independent
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The Royal Society of London |
Experience and Qualifications
Dr. Goldstein has been a Professor of Molecular Genetics and Internal Medicine and the Chairman of the Department of Molecular Genetics at The University of Texas Southwestern Medical Center at Dallas since 1977. Dr. Goldstein is a member of the National Academy of Sciences, the National Academy of Medicine, and the Royal Society of London. He also serves on the Boards of Trustees of The Rockefeller University and the Howard Hughes Medical Institute. Drs. Goldstein and Brown jointly received the Nobel Prize for Physiology or Medicine in 1985 and the U.S. National Medal of Science in 1988.
Dr. Goldstein’s extensive research experience, his distinguished scientific and academic credentials, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his substantial understanding of the Company gained through his service as a director since 1991, led to the board’s decision to nominate Dr. Goldstein for reelection to the board.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Compensation Committee | |
| Corporate Governance and Compliance Committee | 2/2* |
| Technology Committee | 4/4 |
| * | Dr. Goldstein was elected as a member of the Corporate Governance and Compliance Committee on June 14, 2019 and ceased to serve as a member of the Compensation Committee at such time. |
Prior Voting Results—2017
| For | 84.6% |
| Against | 15.4% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 9,000 |
| Options | 38,554 |
| Restricted Stock Units (“RSUs”) | 465 |
| 1 | Biographical information is given, as of April 14, 2020, for each nominee and for each of the other directors whose term of office will continue after the 2020 Annual Meeting. All the nominees are presently directors and were previously elected by the shareholders. None of the corporations or other organizations referred to below with which a director has been or is currently employed or otherwise associated is a parent, subsidiary, or affiliate of the Company. |
10 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
CHRISTINE A. POON

Director since:2010
Age:67
Independent
Other Public Boards
| • | Prudential Financial, Inc. |
| • | The Sherwin-Williams Company |
| • | Royal Philips Electronics |
Experience and Qualifications
Ms. Poon is an Executive-in-Residence in the Department of Management and Human Resources at The Max M. Fisher College of Business at The Ohio State University, where she served as Dean and the John W. Berry, Sr. Chair in Business from 2009 to 2014. Prior to joining Fisher, Ms. Poon spent eight years at Johnson & Johnson, most recently as vice chairman and worldwide chairman of pharmaceuticals. At Johnson & Johnson, she served on the company’s board of directors and executive committee and was responsible for managing the pharmaceutical businesses of the company. Prior to joining Johnson & Johnson, Ms. Poon spent 15 years at Bristol-Myers Squibb Company, a global pharmaceutical company, where she held senior leadership positions including president of international medicines and president of medical devices. Ms. Poon serves on the boards of directors of Prudential Financial, Inc. and The Sherwin-Williams Company and the Supervisory Board of Royal Philips Electronics.
Ms. Poon’s extensive expertise in domestic and international business operations, including sales and marketing and commercial operations, and her deep strategic and operational knowledge of the pharmaceutical industry, led to the board’s decision to nominate Ms. Poon for reelection to the board.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Compensation Committee (Chairperson) | 14/14 |
| Corporate Governance and Compliance Committee | 5/5 |
Prior Voting Results—2017
| For | 84.0% |
| Against | 16.0% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 790 |
| Options | 120,834 |
| RSUs | 465 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 11 / 11 |
BOARD OF DIRECTORS / MEET THE BOARD
P. ROY VAGELOS, M.D.

Director since:1995
Age:90
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The American Philosophical Society |
Experience and Qualifications
Prior to joining Regeneron, Dr. Vagelos was Chairman of the Board and Chief Executive Officer of Merck & Co., Inc., a global pharmaceutical company. He joined Merck in 1975, became a director in 1984, President and Chief Executive Officer in 1985, and Chairman in 1986. Dr. Vagelos retired from all positions with Merck in 1994. Dr. Vagelos served on the board of directors of Theravance, Inc. from 1996 to 2010. Dr. Vagelos is a member of the National Academy of Sciences, the National Academy of Medicine, and the American Philosophical Society. During his tenure as Chairman of Regeneron and previously as Chairman and Chief Executive Officer of Merck, Dr. Vagelos developed an extensive understanding of the complex business, operational, scientific, regulatory, and commercial issues facing the pharmaceutical industry.
Dr. Vagelos’s tenure and experience with the Company and Merck, his extensive knowledge of the pharmaceutical industry, his substantial leadership experience, and his significant understanding of the Company led to the board’s decision to nominate Dr. Vagelos for reelection to the board.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Technology Committee | 4/4 |
Prior Voting Results—2017
| For | 99.5% |
| Against | 0.5% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 675,911 |
| Options | 1,145,495 |
12 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
HUDA Y. ZOGHBI, M.D.

Director since:2016
Age:65
Independent
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The Institute of Medicine |
| • | The American Association for the Advancement of Science |
Experience and Qualifications
Dr. Zoghbi is currently a professor in the departments of Pediatrics, Molecular and Human Genetics, and Neurology and Neuroscience at Baylor College of Medicine, the director of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, and an investigator of the Howard Hughes Medical Institute. She has been elected to the National Academy of Sciences, the Institute of Medicine, and the American Association for the Advancement of Science, and has been awarded numerous recognitions for her work, including the Pearl Meister Greengard Prize, the March of Dimes Prize in Developmental Biology, and the Vanderbilt Prize in Biomedical Science.
Dr. Zoghbi earned her B.Sc. from the American University of Beirut, received her M.D. from Meharry Medical College in Nashville, Tennessee, and completed her pediatrics residency and a joint residency in neurology and pediatric neurology at Baylor College of Medicine, where she then pursued postdoctoral research training in molecular genetics.
Dr. Zoghbi’s extensive research experience and her scientific and academic career and accomplishments led to the board’s decision to nominate Dr. Zoghbi for reelection to the board.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Compensation Committee | 14/14 |
| Technology Committee | 4/4 |
Prior Voting Results—2017
| For | 97.7% |
| Against | 2.3% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 0 |
| Options | 28,465 |
| RSUs | 465 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 13 / 13 |
BOARD OF DIRECTORS / MEET THE BOARD
NOMINEE FOR CLASS III DIRECTOR
FOR ELECTION AT THE 2020 ANNUAL MEETING FOR A TERM EXPIRING AT THE 2021 ANNUAL MEETING
N. ANTHONY COLES, M.D.

Director since:2017
Age:59
Independent
Other Public Boards
Experience and Qualifications
Dr. Coles has served as the Executive Chairman and Chief Executive Officer of Cerevel Therapeutics, LLC, a Bain-portfolio biotechnology company specializing in the development of new therapies for diseases of the central nervous system, since 2019. Previously, from 2014 to 2019, Dr. Coles served as Chief Executive Officer of Yumanity Therapeutics, LLC, a private company focused on transforming drug discovery for neurodegenerative diseases, and continues to serve as the Executive Chairman of the Board. From 2013 to 2014, Dr. Coles served as Chairman and CEO of TRATE Enterprises LLC, a privately held company. Dr. Coles served as President, Chief Executive Officer and Chairman of the Board of Onyx Pharmaceuticals, Inc., a biopharmaceutical company, from 2012 until 2013, having served as its President, Chief Executive Officer, and a member of its board of directors from 2008 until 2012. Prior to joining Onyx in 2008, he was President, Chief Executive Officer, and a member of the board of directors of NPS Pharmaceuticals, Inc., a biopharmaceutical company. Before joining NPS in 2005, he served in various leadership positions in the biopharmaceutical and pharmaceutical industries, including at Merck & Co., Inc., Bristol-Myers Squibb Company, and Vertex Pharmaceuticals Incorporated. In addition to having previously served as a director of Onyx and NPS, he was formerly a director of Laboratory Corporation of America Holdings, Campus Crest Communities, Inc., and CRISPR Therapeutics AG.
Dr. Coles has been a director of McKesson Corporation since April 2014 and serves on the Compensation Committee and the Finance Committee of its board of directors.
The experience of Dr. Coles as a seasoned executive and corporate director with extensive knowledge of highly regulated biopharmaceutical and pharmaceutical companies, as well as his in-depth knowledge and understanding of the regulatory environment in which Regeneron operates, led to the board’s decision to nominate Dr. Coles for reelection to the board.
Board and Committee Membership—2019 Attendance
| Board of Directors | 5/6 |
| Audit Committee | 8/9 |
Prior Voting Results—2017
| For | 99.7% |
| Against | 0.3% |
Regeneron Common Stock Beneficially Owned as of April 14, 2020
| Common Stock | 0 |
| Options | 23,370 |
| RSUs | 465 |
14 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
CLASS III DIRECTORS CONTINUING IN OFFICE
TERM EXPIRES AT THE 2021 ANNUAL MEETING
ARTHUR F. RYAN

Director since:2003
Age:77
Independent
Other Public Boards
Experience and Qualifications
In 2008, Mr. Ryan retired as the Chairman of the Board of Prudential Financial, Inc., one of the largest diversified financial institutions in the world. He served as Chief Executive Officer of Prudential until 2007. Prior to joining Prudential in 1994, Mr. Ryan served as President and Chief Operating Officer of Chase Manhattan Bank since 1990. Mr. Ryan managed Chase’s worldwide retail bank between 1984 and 1990. From 2008 to 2013, Mr. Ryan served as a non-executive director of the Royal Bank of Scotland Group plc. From 2009 to 2019, Mr. Ryan served as a director of Citizens Financial Group, Inc., a retail bank holding company that became publicly traded in 2014, and also served as its lead director, chair of the Compensation and Human Resources Committee, and a member of the Nominating and Corporate Governance Committee.
Mr. Ryan’s substantial leadership experience as a chief executive officer of leading companies in the banking and insurance industries, and his extensive business experience and financial expertise, led the board to conclude that Mr. Ryan should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Audit Committee | 9/9 |
| Corporate Governance and Compliance Committee (Chairman) | 5/5 |
Prior Voting Results—2018
| For | 88.8% |
| Against | 11.2% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 28,300 |
| Options | 52,054 |
| RSUs | 465 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 15 / 15 |
BOARD OF DIRECTORS / MEET THE BOARD
GEORGE L. SING

Director since:1988
Age:70
Independent
Experience and Qualifications
Since 1998, Mr. Sing has been a Managing Director of Lancet Capital, a venture capital investment firm in the healthcare field. In addition, since 2016, Mr. Sing has served as Chief Executive Officer of GanD, Inc., a biomedical drug development company. From 2004 to 2015, Mr. Sing served as Chief Executive Officer of Stemnion, Inc. (currently known as Noveome Biotherapeutics, Inc.), a biomedical company in the regenerative medicine field.
Mr. Sing’s extensive healthcare and financial expertise as a healthcare venture capital investor and biomedical company chief executive officer, his executive leadership experience, and his substantial knowledge of the Company led the board to conclude that Mr. Sing should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Audit Committee (Chairman) | 9/9 |
| Compensation Committee | 14/14 |
Prior Voting Results—2018
| For | 64.0% |
| Against | 36.0% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 129,872 |
| Options | 93,804 |
| RSUs | 465 |
16 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
MARC TESSIER-LAVIGNE, PH.D.

Director since:2011
Age:60
Independent
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The Royal Society of London |
| • | The Royal Society of Canada |
Other Public Boards
Experience and Qualifications
Dr. Tessier-Lavigne has been the President of Stanford University since 2016. Before assuming his role at Stanford, he served as the President of The Rockefeller University and a Carson Family Professor and head of the Laboratory of Brain Development at The Rockefeller University from 2011. Previously, he served as Executive Vice President and Chief Scientific Officer at Genentech, Inc., which he joined in 2003. He was a professor at Stanford University from 2001 to 2003 and at the University of California, San Francisco from 1991 to 2001. Dr. Tessier-Lavigne is a member of the National Academy of Sciences, the National Academy of Medicine, and a fellow of the Royal Societies of London and Canada. Dr. Tessier-Lavigne is a member of the Board of Directors of Denali Therapeutics Inc., and previously served on the board of directors of Pfizer Inc., Agios Pharmaceuticals, Inc., and Juno Therapeutics, Inc.
Dr. Tessier-Lavigne’s distinguished scientific and academic background, and his significant industry experience, including experience in senior scientific leadership roles at a leading biopharmaceutical company, led the board to conclude that Dr. Tessier-Lavigne should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Technology Committee | 4/4 |
Prior Voting Results—2018
| For | 94.8% |
| Against | 5.2% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 1,187 |
| Options | 66,304 |
| RSUs | 465 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 17 / 17 |
BOARD OF DIRECTORS / MEET THE BOARD
CLASS I DIRECTORS CONTINUING IN OFFICE
TERM EXPIRES AT THE 2022 ANNUAL MEETING
BONNIE L. BASSLER, PH.D.

Director since:2016
Age:57
Independent
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The American Academy of Arts and Sciences |
| • | The Royal Society of London |
| • | The American Philosophical Society |
Other Public Boards
Experience and Qualifications
Dr. Bassler is currently the Chair of the Department of Molecular Biology and the Squibb Professor in Molecular Biology at Princeton University, and a Howard Hughes Medical Institute Investigator. Dr. Bassler has previously served as the President of the American Society for Microbiology, as well as on the boards for the American Association for the Advancement of Science, the National Science Foundation, and the American Academy of Microbiology. She has been elected to the National Academy of Sciences, the American Academy of Arts and Sciences, the Royal Society of London, and the American Philosophical Society, and has received many scientific honors, including a MacArthur Foundation Fellowship, the Lounsbery Award, and the Shaw Prize for Life Science and Medicine. Dr. Bassler received her B.Sc. from the University of California, Davis, and her Ph.D. in Biochemistry from Johns Hopkins University. She served as a Postdoctoral Fellow and Research Scientist at the Agouron Institute in La Jolla, California, before becoming a faculty member at Princeton University. Dr. Bassler served as a director of Sanofi from November 2014 to July 2016 and currently serves on the board of directors of Kaleido Biosciences, Inc.
Dr. Bassler’s extensive research experience and her scientific and academic career and accomplishments, as well as her experience as a corporate director, led the board to conclude that Dr. Bassler should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Corporate Governance and Compliance Committee | 5/5 |
| Technology Committee | 4/4 |
Prior Voting Results—2019
| For | 75.4% |
| Against | 24.6% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 0 |
| Options | 28,465 |
| RSUs | 465 |
18 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
MICHAEL S. BROWN, M.D.

Director since:1991
Age:79
Independent
Scientific Society Memberships
| • | The National Academy of Sciences |
| • | The National Academy of Medicine |
| • | The Royal Society of London |
Experience and Qualifications
Dr. Brown holds the Distinguished Chair in Biomedical Sciences, a position he has held since 1989, and is a Regental Professor of Molecular Genetics and Internal Medicine, and the Director of the Jonsson Center for Molecular Genetics, at The University of Texas Southwestern Medical Center at Dallas, positions he has held since 1985. Drs. Brown and Goldstein jointly received the Nobel Prize for Physiology or Medicine in 1985 and the U.S. National Medal of Science in 1988. Dr. Brown is a member of the National Academy of Sciences, the National Academy of Medicine, and Foreign Member of the Royal Society of London. Dr. Brown retired as a member of the board of directors of Pfizer Inc. in 2012.
Dr. Brown’s distinguished scientific and academic background, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his significant industry experience gained through his service on the board of directors of the Company and of a leading pharmaceutical company, led the board to conclude that Dr. Brown should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 6/6 |
| Corporate Governance and Compliance Committee | 5/5 |
| Technology Committee (Chairman) | 4/4 |
Prior Voting Results—2019
| For | 70.7% |
| Against | 29.3% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Common Stock | 17,349 |
| Options | 38,804 |
| RSUs | 465 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 19 / 19 |
BOARD OF DIRECTORS / MEET THE BOARD
LEONARD S. SCHLEIFER, M.D., PH.D.

Director since:1988
Age:67
Experience and Qualifications
Dr. Schleifer founded the Company in 1988, has been a Director and its President and Chief Executive Officer since its inception, and served as Chairman of the Board from 1990 through 1994. Dr. Schleifer, together with Regeneron’s founding scientist, Dr. Yancopoulos, built and has managed the Company over the past 32 years. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology. With over 30 years of experience as Chief Executive Officer of the Company, Dr. Schleifer brings to the board an incomparable knowledge of the Company, significant leadership experience, and an in-depth understanding of the complex research, drug development, and business issues facing companies in the biopharmaceutical industry.
Dr. Schleifer’s significant industry and leadership experience, as well as his extensive knowledge of the Company, led the board to conclude that Dr. Schleifer should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 5/6 |
| Technology Committee | 4/4 |
Prior Voting Results—2019
| For | 83.8% |
| Against | 16.2% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Class A Stock | 1,726,565 |
| Common Stock | 580,315 |
| Options | 1,820,844 |
20 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / MEET THE BOARD
GEORGE D. YANCOPOULOS, M.D., PH.D.

Director since:2001
Age:60
Scientific Society Memberships
Experience and Qualifications
Dr. Yancopoulos joined Dr. Schleifer in 1989 as founding scientist of the Company, and together they built and have managed the Company since then. Dr. Yancopoulos is currently President and Chief Scientific Officer, and has served on the board since 2001.
He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and/or developer of the seven FDA-approved drugs the Company has developed, EYLEA®(aflibercept) Injection, Praluent®(alirocumab), Dupixent®(dupilumab), Kevzara®(sarilumab), Libtayo®(cemiplimab), ZALTRAP®(ziv-aflibercept) Injection for Intravenous Infusion, and ARCALYST®(rilonacept) Injection for Subcutaneous Use, as well as of its foundation technologies, including the TRAP technology,VelociGene®, andVelocImmune®. As one of the few members of the National Academy of Sciences from industry and as an author of a substantial number of scientific publications, Dr. Yancopoulos has a distinguished record of scientific expertise. Dr. Yancopoulos also brings to the board his experience in building and managing the Company, his in-depth knowledge of the Company’s technologies and research and development programs, and his proven track-record for envisioning successful long-term strategic directions and opportunities.
Dr. Yancopoulos’s significant industry and scientific experience, as well as his extensive knowledge of the Company, led the board to conclude that Dr. Yancopoulos should serve as a director.
Board and Committee Membership—2019 Attendance
| Board of Directors | 5/6 |
| Technology Committee | 4/4 |
Prior Voting Results—2019
| For | 83.7% |
| Against | 16.3% |
Regeneron Securities Beneficially Owned as of April 14, 2020
| Class A Stock | 42,750 |
| Common Stock | 1,105,698 |
| Options | 1,371,988 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 21 / 21 |

The board has a standing Audit Committee, Compensation Committee, and Corporate Governance and Compliance Committee, each of which is comprised entirely of independent directors. The Corporate Governance and Compliance Committee is responsible for reviewing and recommending for the board’s selection candidates to serve on our board of directors and for overseeing all aspects of the Company’s compliance program other than financial compliance. The board also has a standing Technology Committee. The board has adopted charters for the Audit Committee, Compensation Committee, Corporate Governance and Compliance Committee, and Technology Committee, current copies of which are available on our website atwww.regeneron.comunder the “Corporate Governance” heading on the “Investors & Media” page.
We show below information on the membership, key functions, and number of meetings of each board committee during 2019.
| AUDIT COMMITTEE | ||||
 | ||||
George L. Sing,Chairman N. Anthony Coles, M.D. Arthur F. Ryan Number of Meetings Held in 2019 9 | • | Select the independent registered public accounting firm, review and approve its engagement letter, and monitor its independence and performance. | ||
| • | Review the overall scope and plans for the annual audit by the independent registered public accounting firm. | |||
| • | Approve performance of non-audit services by the independent registered public accounting firm and evaluate the performance and independence of the independent registered public accounting firm. | |||
| • | Review and approve the Company’s periodic financial statements and the results of the year-end audit. | |||
| • | Review and discuss the adequacy and effectiveness of the Company’s accounting and internal control policies and procedures. | |||
| • | Evaluate the internal audit process for establishing the annual audit plan; review and approve the appointment and replacement of the Company’s Chief Audit Executive, if applicable, and any outside entities providing internal audit services and evaluate their performance on an annual basis. | |||
| • | Review the independent registered public accounting firm’s recommendations concerning the Company’s financial practices and procedures. | |||
| • | Oversee the Company’s risk management program. | |||
| • | Discuss with management the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures. | |||
| • | Establish procedures for the receipt, retention, and treatment of complaints received by the Company regarding accounting, internal accounting controls, or auditing matters and for the confidential, anonymous submission by employees of concerns regarding questionable accounting or auditing matters. | |||
| • | Review and approve any related person transaction. | |||
| • | Prepare an annual report of the Audit Committee for inclusion in the Company’s proxy statement. | |||
 | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / BOARD COMMITTEES
| COMPENSATION COMMITTEE | |||||||
 | |||||||
Christine A. Poon, Chairperson Joseph L. Goldstein, M.D. George L. Sing Huda Y. Zoghbi, M.D. Number of Meetings Held in 2019 14 | • | Evaluate the performance of the Chief Executive Officer and other executive officers of the Company. | |||||
| • | Recommend compensation for the Chief Executive Officer for approval by the non-employee members of the board of directors. | ||||||
| • | Approve compensation for other executive officers. | ||||||
| • | Approve the total compensation budget for all Company employees. | ||||||
| • | Oversee the Company’s compensation and benefit philosophy and programs generally. | ||||||
| • | Review and approve annually the corporate goals and objectives applicable to the compensation of the Chief Executive Officer and the goals and objectives of the Company’s executive compensation programs. | ||||||
| • | Review and approve the Compensation Discussion and Analysis to be included in the Company’s proxy statement. | ||||||
| • | Prepare an annual report of the Compensation Committee for inclusion in the Company’s proxy statement. | ||||||
 | |||||||
11
Proposal No. 1: Election of DirectorsCORPORATE GOVERNANCE AND COMPLIANCE COMMITTEE
|
 | |||||||
Arthur F. Ryan, Chairman Bonnie L. Bassler, Ph.D. Michael S. Brown, M.D. Joseph L. Goldstein, M.D. Christine A. Poon Number of Meetings Held in 2019 5 | • | Identify qualified individuals to become members of the board and recommend such candidates to the board. | |||||
| • | Assess the functioning of the board and its committees and make recommendations to the board concerning the appropriate size, function, and needs of the board. | ||||||
| • | Review, and make recommendations to the board regarding, non-employee director compensation. | ||||||
| • | Make recommendations to the board regarding corporate governance matters and practices. | ||||||
| • | Oversee all aspects of the Company’s comprehensive compliance program other than financial compliance. | ||||||
| • | Oversee the Company’s key corporate responsibility initiatives and conduct a periodic review of environmental, social, and governance matters. | ||||||
 | |||||||
 | |||||||
12Key Functions
Proposal No. 1: Election of Directors
|
Michael S. Brown, M.D., Chairman Bonnie L. Bassler, Ph.D. Joseph L. Goldstein, M.D. Marc Tessier-Lavigne, Ph.D. P. Roy Vagelos, M.D. Huda Y. Zoghbi, M.D. Leonard S. Schleifer, M.D., Ph.D.+ George D. Yancopoulos, M.D., Ph.D.+ Number of Meetings Held in 2019 4 | • | Review and evaluate the Company’s research and clinical development programs, plans, and policies. | ||
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 23 / 23 |

Pursuant to the Company’s Certificate of Incorporation, the board of directors is divided into three classes, denominated Class I, Class II, and Class III, with members of each class holding office for staggered three-year terms. There are currently four members in Class I, five members in Class II, and three members in Class III. As discussed above, the board of directors has determined that it would be in the best interests of the Company and its shareholders to apportion the directorships equally among the three classes of the board so that each class consist of four directors. This equal apportionment will be effected at the 2020 Annual Meeting if all of the nominees for Class II Director and Class III Director are elected at the 2020 Annual Meeting. The respective terms of the directors expire (in all cases, subject to the election and qualification of their successors and to their earlier death, resignation, or removal) as follows:
| • | The terms of the Class I Directors expire at the 2022 Annual Meeting; | |||
 | ||||
• | ||||
 | ||||
 | ||||
13
Proposal No. 1: Election of Directors
 | 2021 Annual Meeting. |
14
Proposal No. 1: ElectionDr. Coles is Sanofi’s designee to the board of Directors
![]()
Corporate Governancedirectors in accordance with the Amended and Restated Investor Agreement between us and Sanofi described under “Certain Relationships and Related Transactions—Transactions with Related Persons—Amended and Restated Investor Agreement with Sanofi; 2018 Letter Agreement.”
OverviewBOARD MEETINGS AND ATTENDANCE OF DIRECTORS
Regeneron is committed to good corporate governance, which we believe promotes
The board held six regular meetings in 2019. All directors attended at least 75% of the long-term intereststotal number of shareholders, strengthens the accountabilitymeetings of the board and committees of the board on which they served. According to the Regeneron Board of Directors Corporate Governance Guidelines, board members are expected to attend the Company’s Annual Meeting of Shareholders. All of the directors
and management, and helps build trust then in the Company. The following chart summarizes key information regardingoffice (other than Dr. Coles, who was excused due to an unplanned personal matter) attended our corporate governance.
| ||
| ||
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
2019 Annual |
| |
|
| |
|
| |
|
| |
|
| |
*AsofApril 14, 2016.
Procedures Relating to NomineesPROCEDURES RELATING TO NOMINEES; BOARD SUCCESSION PLANNING
The Corporate Governance and Compliance Committee will consider a nominee for election to the board of directors recommended by a shareholder of record if the shareholder submits the nominationrecommendation in compliance with the requirements of our by-laws and the Guidelines Regarding Director Nominations, which are available on our website atwww.regeneron.comunder the "Corporate Governance"“Corporate Governance” heading on the "Investors“Investors & Media"Media” page.
In considering potential candidates for the board of directors, the Corporate Governance and Compliance Committee considers factors such as whether or not a potential candidate: (1) possesses relevant expertise; (2) brings skills and experience complementary to those of the other members of the board; (3) has sufficient time to devote to the affairs of the Company; (4) has demonstrated excellence in his or her field; (5) has the ability to exercise sound business judgment; (6) has the commitment to rigorously represent the long-term interests of the Company'sCompany’s shareholders; (7) possesses a diverse background and experience, including with respect to race, age, and gender; and (8) such other factors as the Corporate Governance and Compliance Committee may determine from time to time.
Candidates for director are reviewed in the context of the current composition of the board of directors, the operating requirements of the Company, and the long-term interests of
shareholders. In conducting the assessment, the Committee considers the individual'sindividual’s independence, experience, skills, background, and diversity, including with respect to race, age, and gender, along with such other factors as it deems appropriate, given the current needs of the board and the Company to maintain a balance of knowledge, experience, and capabilities. When recommending a slate of director nominees each year, the Corporate Governance and Compliance Committee reviews the current composition of the board of directors in order to recommend a slate of directors who, with the continuing directors, will provide the board with the requisite diversity of skills, expertise, experience, and viewpoints necessary to effectively fulfill its duties and responsibilities.
24 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
BOARD OF DIRECTORS / BOARD GOVERNANCE
In the case of an incumbent director whose term of office is set to expire, the Corporate Governance and Compliance Committee reviews such director'sdirector’s overall service to the Company during the director'sdirector’s term and also considers the director'sdirector’s interest in continuing as a member of the board. In the case of a new director candidate, the Corporate Governance and Compliance Committee also reviews whether the nominee is "independent,"“independent,” based on our Corporate Governance Guidelines, applicable listing standards of the NASDAQNasdaq Stock Market LLC, and applicable SEC and other relevant rules and regulations, if necessary.
The Corporate Governance and Compliance Committee may employ a variety of methods for identifying and evaluating
15
Corporate Governance
nominees for the board of directors. TheIn addition, the Corporate Governance and Compliance Committee may consider candidates recommended by other directors, management, search firms, shareholders, or other sources. When conducting searches for new directors, the Corporate Governance and Compliance Committee will take reasonable steps to include diverse candidates in the pool of nominees and any search firm will affirmatively be instructed to seek to include diverse candidates. Candidates recommended by shareholders will be evaluated on the same basis as candidates recommended by our directors or management or by third party search firms or other sources. Candidates may be evaluated at regular or special meetings of the Corporate Governance and Compliance Committee.
The Corporate Governance and Compliance Committee seeks to Remove Directorsensure that our board of directors as a whole possesses the mix of skills and experiences to provide effective oversight and guidance to management to execute on the Company’s long-term strategy. The Committee also considers succession planning for Causeboard and committee chairs for purposes of continuity and to Call Special Shareholder Meetingmaintain relevant expertise and depth of experience.

BOARD AND COMMITTEE SELF-ASSESSMENTS
On an annual basis, the board of directors, the Audit Committee, the Compensation Committee, and the Corporate Governance and Compliance Committee conduct self-assessments to ensure effective performance and to identify opportunities for improvement. As the first step in the self-assessment process, directors complete a comprehensive questionnaire, which asks them to consider various topics related to board and committee composition, structure, effectiveness, and responsibilities, as well as satisfaction with the schedule, materials, and discussion topics. Each committee, as well as the board as a whole, then reviews and assesses the responses and presents its findings and recommendations to the board of directors. The results of the assessments are then discussed by the board of directors and the respective committees in executive session, with a view toward taking action to address any issues presented. Results requiring additional consideration are addressed at subsequent board and committee meetings, where appropriate.
Annual Self-Assessment Process
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 25 / 25 |
BOARD OF DIRECTORS / BOARD GOVERNANCE
Regeneron's
While this formal self-assessment is conducted on an annual basis, directors share perspectives, feedback, and suggestions year-round, both inside and outside the boardroom.
SHAREHOLDER RIGHTS TO REMOVE DIRECTORS FOR CAUSE AND TO CALL SPECIAL SHAREHOLDER MEETING
Regeneron’s charter documents give shareholders the rights to (i) remove directors for cause by an affirmative vote of at least 80% of the outstanding shares of all classes of capital stock entitled to vote for directors; and (ii) call a special shareholder meeting upon the written request of at least 25% of the total number of votes entitled to be cast by shareholders.
Shareholder Communications with DirectorsDIRECTOR INDEPENDENCE
The Company has established a process for shareholders to send communications to the members of the board of directors. Shareholders may send such communications by mail addressed to the full board, a specific member or members of the board, or a particular committee of the board, at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707,
Attention: Corporate Secretary. All such communications will be opened by our Corporate Secretary for the sole purpose of determining whether the contents represent a message to our directors. Any contents that are not in the nature of advertising, promotions of a product or service, or patently offensive material will be forwarded promptly to the addressee. In the case of communications to the board or any individual director or group or committee of directors, the Corporate Secretary will make sufficient copies of the contents to send to such director or each director who is a member of the group or committee to which the envelope is addressed.
The board has a standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), a Compensation Committee, and a Corporate Governance and Compliance Committee, each of which is comprised entirely of independent directors. The Corporate Governance and Compliance Committee is responsible for reviewing and recommending for the board's selection candidates to serve on our board of directors and for overseeing all aspects of the Company's compliance program other than financial compliance. The board also has a standing Technology Committee. The board has adopted charters for the Audit Committee, Compensation Committee, Corporate Governance and Compliance Committee, and Technology Committee, current copies of which are available on our website atwww.regeneron.com under the "Corporate Governance" heading on the "Investors & Media" page.
16
Corporate Governance
We show below information on the membership, key functions, and number of meetings of each board committee during 2015.
|
|
17
Corporate Governance
|
| |||||
| ||||||
|
| |||||
|
| |||||
+Dr. Tessier-Lavigne resigned as Chairman and a member of the Compensation Committee effective as of April 1, 2016. Ms. Poon was appointed as Chairperson of the Compensation Committee effective as of Dr. Tessier-Lavigne's resignation date.
*Ex OfficioMember.
The board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers, and directors. You can find links to this code on our website atwww.regeneron.com under the "Corporate Governance" heading on the "Investors & Media" page. We may satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding
an amendment to, or a waiver from, a provision of our code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or controller, or persons performing similar functions, by posting such information on our website where it is accessible through the same link noted above.
18
Corporate Governance
The board of directors has determined that each of the following currently serving directors is independent as defined in the listing standards of The NASDAQNasdaq Stock Market LLC and our Corporate Governance Guidelines: Charles A. Baker,Bonnie L. Bassler, Ph.D., Michael S. Brown, M.D., N. Anthony Coles, M.D., Joseph L. Goldstein, M.D., Christine A. Poon, Arthur F. Ryan, George L. Sing, and Marc Tessier-Lavigne, Ph.D., and Huda Y. Zoghbi, M.D. These individuals are affiliated with numerous educational institutions, hospitals, charities, and corporations, as well as civic organizations and professional associations. The board of directors considered each of these relationships and determined that none of these relationships conflicted with the interests of the Company or would impair their independence or judgment. The board conducts executive sessions of independent directors following each regularly scheduled board meeting.
The board of directors has determined that each of the current members of the Audit Committee, Messrs. Baker, Ryan and Sing and Dr. Coles, qualifies as an "audit“audit committee financial expert"expert” as that term is defined in Item 407(d)(5)(ii) of Regulation S-K under the Exchange Act, meets the required standards for independence set forth in Rule 10A-3(b)(1) under the Exchange Act,by SEC rules, and is independent as defined for audit committee members in the listing standards of The NASDAQNasdaq Stock Market LLC.LLC and SEC rules.
In addition, the board of directors has determined that each of the current members of the Compensation Committee, Ms. Poon, Messrs. Baker andMr. Sing, and Dr. Goldstein,Zoghbi, meets the additional independence criteria applicable to compensation committee members under the listing standards of The NASDAQNasdaq Stock Market LLC and qualifies as a "Non-Employee Director"“Non-Employee Director” pursuant to Rule 16b-3 under the Securities Exchange Act andof 1934, as an "outside director" within the meaning of Section 162(m) of the Internal Revenue Code.amended (the “Exchange Act”).
Board Leadership and Role in Risk OversightBOARD LEADERSHIP AND ROLE IN RISK OVERSIGHT
The board of directors recognizes that one of its key responsibilities is to establish and evaluate an appropriate leadership structure for the board so as to provide effective oversight of management. Since 1995, the board has separated the roles of the Chief Executive Officer and the Chairman of the Board, with Dr. Vagelos serving as Chairman and Dr. Schleifer serving as President and Chief Executive Officer. Dr. Vagelos'sVagelos’s extensive leadership experience, his business acumen, and his deep understanding of the healthcare industry have made him an invaluable resource to both the board and Dr. Schleifer. The board has determined that this leadership structure is appropriate for the Company at this time.
The board executes its oversight responsibility for risk management directly and through its Committees,committees, as follows:
•The Audit Committee oversees the Company's risk management program. The Company's Chief Audit Executive,
| • | The Audit Committee oversees the Company’s risk management program. The risk management program focuses on the most significant risks the Company faces. The Company’s Chief Audit Executive, who reports independently to the Committee, facilitates the risk management program. Audit Committee meetings include discussions of specific risk areas throughout the year, including, among others, those relating to cybersecurity, and reports from the Chief Audit Executive on the Company’s enterprise risk profile on an annual basis. |
| • | The Compensation, Corporate Governance and Compliance, and Technology Committees oversee risks associated with their respective areas of responsibility. As part of its overall review of the Company’s compensation policies and practices, the Compensation Committee generally considers the risks associated with these policies and practices. The Corporate Governance and Compliance Committee oversees all aspects of the Company’s comprehensive compliance program other than financial compliance and considers legal and regulatory compliance risks. The Technology Committee considers risks associated with our research and development programs. |
26 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
who reports independently to the Committee, facilitates the risk management program. Audit Committee meetings include discussions of specific risk areas throughout the year, as well as annual reports from the Chief Audit Executive on the Company's enterprise risk profile.
•
The Compensation, Corporate Governance and Compliance, and Technology Committees oversee risks associated with their respective areas of responsibility. As part of its overall review of the Company's compensation policies and practices, the Compensation Committee generally considers the risks associated with these policies and practices. The Corporate Governance and Compliance Committee oversees all aspects of the Company's comprehensive compliance program other than financial compliance and considers legal and regulatory compliance risks. The Technology Committee considers risks associated with our research and development programs.BOARD OF DIRECTORS /
BOARD GOVERNANCE
Board Meetings and Attendance of DirectorsEXECUTIVE COMPENSATION PROCESSES AND PROCEDURES; ROLE OF COMPENSATION CONSULTANTS
The board held five regular meetings and two special meetings in 2015. All directors attended more than 75% of the total number of meetings of the board and committees of the board on which they served. According to the Regeneron Board of Directors Corporate Governance Guidelines, board members are expected to attend the Company's Annual Meeting of Shareholders. All of the directors attended our 2015 Annual Meeting of Shareholders, with one director participating through electronic conferencing.
Executive Compensation Processes and Procedures; Role of Compensation Consultants
The Compensation Committee is responsible for overseeing the Company'sCompany’s general compensation objectives and programs. We describe below under “Compensation-Related Matters — Compensation Discussion and Analysis — Our Compensation Processes” the role of the Compensation Committee, as well as the role of our executive officers, in decisions regarding executive compensation (particularly with respect to our Named Officers referred to below under "Executive Compensation") below under "Executive Compensation – Compensation Discussion and Analysis – Section 3 – Executive Compensation Process and Considerations – Overview."
19Officers).
Corporate Governance
As discussed in greater detail under "Executive Compensation – Compensation Discussion and Analysis – Section 3 – Executive Compensation Process and Considerations," theThe Compensation Committee has the sole authority to retain its own third-party compensation consultants, and in 20152019 utilized the services of Frederic W. Cook & Co., Inc. ("(“Frederic W. Cook & Co."”), aan independent compensation consultant. Advice and recommendations provided by Frederic W. Cook & Co. may relate to both executive compensation (discussed under "Executive Compensation"in the section “Compensation-Related Matters” below) and director compensation matters (discussed under " – Compensationin the subsection “Compensation of Directors"Directors” below). In addition, managementAs discussed further below, the Corporate Governance and Compliance Committee has adopted a policy requiring the annual review of non-employee director compensation by an independent compensation consultant and separately engaged Frederic W. Cook & Co. for that purpose.
Management also retains another compensation consultant for its own use. In 2015,2019, management used the services of Radford, a compensation consultant focused on the technology and life sciences sectors. Radford provided various consulting services to us, including analyzing the competitiveness of specific compensation programs; preparing surveys of competitive pay practices (including the 2015 Radford Global Life Sciences SurveyMarket Composite Data discussed in this proxy statement)“Compensation-Related Matters — Compensation Discussion and Analysis” below); and assisting management in the development and analysis of executive compensation recommendations. Reports prepared by Radford that relate to executive compensation may also be shared with the Compensation Committee.Committee, the full board, or another committee of the board.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 27 / 27 |

Compensation of DirectorsCOMPENSATION OF DIRECTORS
Overview
| OVERVIEW |
The general philosophy we have applied to compensation of our non-employee directors and the Chairman of the Board is similar to the executive compensation philosophy outlined in the Compensation Discussion and Analysis“Compensation-Related Matters” section of this proxy statement. This philosophy, places anincluding its emphasis on equity compensation, inis consistent with the formCompany’s long-term business orientation and has helped ensure alignment of stock options, which reward growth in stock price and align the directors'directors’ interests with those of our shareholders by providing value to the directors only if there is future stock price appreciation and not rendering any value to the directors if the stock price declines below the applicable exercise price. Similar to executive compensation, the emphasis on long-term incentives in the form of stock options has been a consistent part of Regeneron'sRegeneron shareholders.
Non-employee director compensation philosophy and preceded the significant appreciation in Regeneron's stock price that began in early 2011. As discussed in greater detail below, the board of directors voluntarily reduced the number of shares underlying the three most recentmatters are subject to annual stock option awards to the non-employee directors and the Chairman of the Board by 15% each time, consistent with the reductions in annual awards to executive officers implemented in December 2013, 2014, and 2015.
review. The Corporate Governance and Compliance Committee makes recommendations to the board of directors regarding, and the board of directors determines, the compensation of non-employee directors. The Corporate Governance and
Compliance Committee evaluates the appropriate level and form of compensation for non-employee directors at least annually and recommends changes to such compensation to the board of directors when appropriate. Directors who are Company employees receive no additional compensation for serving on our board of directors or its committees. In determining compensation recommendations for the non-employee directors, the Corporate Governance and Compliance Committee considers, among other things, the qualifications, expertise, and demands on our directors, practices of similar companies in the biotechnology industry (including the Peer Group2), and any recommendationscomparative information provided by Frederic W. Cook & Co. In addition, since November 2018, the Corporate Governance and Compliance Committee has required the annual review of non-employee director compensation by an independent compensation consultant, and has engaged Frederic W. Cook & Co. for this purpose in each of the last two years.
The process governing the compensation consultantsarrangements of the Committee or management. Chairman of the Board is described under “Compensation Arrangements of the Chairman of the Board of Directors” below.
The recent changescurrent compensation program for our non-employee directors and the Chairman of the Board (which has been effective for our non-employee directors and the Chairman of the Board since January 2019 and December 2018, respectively, and is further described below) is referred to in this section as the “Current Compensation Program.”3
| NON-EMPLOYEE DIRECTOR COMPENSATION PHILOSOPHY |
Our philosophy for non-employee director compensation is simple: to attract the most highly qualified directors with a diverse skillset who will serve as stewards of the Company’s long-term prospects and scientific focus. With this in mind, our non-employee director compensation program described below were adopted based on such considerations.
Cash Feesemphasizes equity compensation primarily in the form of stock options, which reward increases in stock price, over cash fees. The board of directors believes that this emphasis is consistent with the Company’s long-term business orientation and Matching Gifthas helped ensure alignment of directors’ interests with those of Regeneron shareholders. Under the Current Compensation Program,
A we have utilized value-denominated equity compensation awards (granted in the form of stock options and a relatively small percentage of RSUs) for our non-employee directors. This feature of the Current Compensation Program is meant to, among other things, ensure greater stability in reported non-employee director receivescompensation on a year-over-year basis. The board of directors believes that the Current Compensation Program is consistent with Regeneron’s philosophy for non-employee director compensation.
| 2 | See “Compensation-Related Matters—Compensation Discussion and Analysis—Our Compensation Processes—Peer Data” below for a list of the companies included in our Peer Group. |
| 3 | The Current Compensation Program was implemented following the previously disclosed settlement of two shareholder derivative actions (the “Settlement”) and complies with the terms of the Settlement. |
28 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
| CASH FEES AND MATCHING GIFT PROGRAM |
In 2019, each non-employee director received an annual retainer of $55,000$90,000 and an annual committee retainer of $10,000 for each standing committee of the Company’s board of directors on which the director serves.served. In 2015, the Chairmanaddition, each chairperson of the Audit Committeeeach standing committee received an additional retainer of $5,000 (increased to $10,000 in the aggregate starting in 2016 as a result of the change described in the following sentence). Starting in 2016, the board of directors approved an annual retainer of $10,000 for each chairperson$10,000. Compared to cash compensation of non-employee directors in our Peer Group, the standing committees of the Company's board of directors, which is in addition to the2019 annual retainer payable to all non-employee directorsfor board service was below the 75th percentile and the annual retainer payable in respect of service as a director on each standingadditional retainers provided to our committee (which remained unchanged). chairpersons were below the median.4
Non-employee directors are reimbursed for their actual expenses incurred in connection with their activities as directors, which included travel, hotel, and food and entertainment expenses. In addition, directors are eligible to participate in the Regeneron Matching Gift Program, which was adopted effective January 1, 2013 and is also available to eligible employees. Under this program, the Company matches contributions made by directors and employees to eligible tax-exempt organizations. Effectiveorganizations up to an annual maximum amount of $5,000 per director or employee.
| ANNUAL EQUITY AWARDS |
2019 and 2020 Equity Awards
The January 1, 2014,2019 and January 2020 annual equity awards to our non-employee directors were made in accordance with the maximum annual match has been increased to $5,000 (from $2,000) per person.
Annual Stock Option Awards
Pursuant toCurrent Compensation Program and complied with the terms ofrespective limits imposed by the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan and resolutionsthe Settlement. If approved by the shareholders at the 2020 Annual Meeting, such limits will also be incorporated into the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. See “Proposal No. 3: Second Amendment and Restatement of Long-Term Incentive Plan” for more information on these limits and other terms of this Plan.
With respect to each of the Compensation Committee adopted on December 16, 2014January 2019 and December 16, 2015, eachJanuary 2020 annual equity awards, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) determined that the targeted aggregate grant date fair value of such equity awards would be set at $600,000 per non-employee director receivesand consist of stock options with a grant date fair value of $480,000 (or 80% thereof) and RSUs with a grant date fair value of $120,000 (or 20% thereof). The January 2019 annual equity awards to our non-employee directors are shown in the table below. On January 2, 2020, each of the then-serving nine non-employee directors received an automaticequity award comprised of stock options representing 4,361 shares of common stock (with a grant date fair value of $480,281) and 320 RSUs (with a grant date fair value of $119,718). The aggregate grant date fair value of the January 2020 equity awards was $599,999 per non-employee director.
Similar to the process undertaken in respect of the January 2019 annual equity awards, the Corporate Governance and Compliance Committee recommended the approval of, and the board of directors approved, the terms of the January 2020 annual equity awards after consideration of the review, analysis, and recommendations of Frederic W. Cook & Co. Such analysis focused on, among other matters, the market practices of companies in our Peer Group, other relevant industry and market data points, Regeneron’s non-employee director compensation philosophy (including its emphasis on long-term incentives), and the terms of the Settlement.
Terms of Equity Awards
The exercise price of a non-employee director stock option to purchase common stock on the first business day of each year, with an exercise priceis equal to the fair market value of a share of common stock on the date of grant (determined for so long as our common stock is listed on the NASDAQ Global Select Market, as the average of the high and low sales price per share of common stock on the NASDAQNasdaq Global Select Market on the date of grant or, if
20
Corporate Governance
such date is not a trading day, on the last preceding date on which there was a sale of the Company'sCompany’s common stock on the NASDAQNasdaq Global Select Market). These
Under the Current Compensation Program, a pro-rata portion of each equity award (i.e., each stock options become exercisable asoption and RSU
| 4 | Based on information reported by our Peer Group companies in 2019. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  /29 /29 |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
award) equal to one-thirdthe portion of one year that has passed from its date of grant vests on the date of the sharesCompany’s first annual shareholder meeting following the date of grant, and the remaining portion vests on the first anniversary of the date of grant in eachgrant. The RSU awards contain mandatory deferral provisions, according to which the shares underlying the RSUs will generally not be delivered to the non-employee director until the earliest of (i) the termination of the three subsequent calendar years,non-employee director’s service as a member of the board, (ii) the seventh anniversary of the RSU grant date, and (iii) the date of a change in control (as defined in the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan). A non-employee director may, subject to compliance with applicable tax rules, elect in writing a maximum deferral period longer than the seventh anniversary of the grant date. Other than as discussed below, the vesting of equity awards is generally subject to continued service on the board, and stock option awards generally expire ten years following the date of grant. In 2015, similar to the reductions in annual awards to executive officers and other employees discussed under "Executive Compensation" below, the Compensation Committee reduced the automatic grant of stock options to our non-employee directors by 15%, from 10,838 shares to 9,212 shares of common stock underlying each such stock option. Similar to the rationale for the reductions in annual awards to executive officers and other employees discussed under "Executive Compensation" below, the impetus for the reductions in the automatic grants to the non-employee directors was to reduce the potential dilutive impact of these grants; the reductions also took into account the increase in the Company stock price since the prior years' awards, with the resulting increases in the grant date fair values of the automatic grants (as determined according to the Black-Scholes model for valuing stock options). The reduced grants to our non-employee directors were made on January 4, 2016. This decrease constituted the third consecutive double-digit percentage decrease in the annual grant of stock options to our non-employee directors, in each case following high stock appreciation, consistent with the reductions in annual awards to executive officers discussed under "Executive Compensation" below.
To the extent they remain unvested and outstanding, stock optionsequity awards granted to a non-employee director continue to vest following the retirement of that director provided applicable conditions relating to the length of the director'sdirector’s service and the director'sdirector’s age have been met. If a non-employee director'sdirector’s service as a member of the board is terminated as a result of his or her death, all of the director's stock optionsdirector’s equity awards will immediately vest in full.
To the extent they remain unvested and outstanding, stock optionsequity awards granted to non-employee directors become fully vested automatically upon a change ofin control of the Company. Each non-employee director has the right to nullify this acceleration of vesting, in whole or in part, if it would cause the director to pay excise taxes under the requirements of the Internal Revenue Code.
Stock Option Awards to New Directors
| EQUITY AWARDS TO NEW DIRECTORS |
Starting in 2016, each newUnder the Current Compensation Program, any newly elected non-employee director will receive an initial stock optionequity award to purchase a number of shareswith an aggregate grant date fair value equal to 5/3rds of the numberaggregate grant date fair value of shares of common stock
underlying the most recent regular annual stock optionequity award to a non-employee director; and, with respect to the annual stock optionequity award to a non-employee director in respect of the first year of his or her service, the numberaggregate grant date fair value of shares of common stock underlying such annual award will be prorated based on the date as of which the non-employee director first becomes a member of the board of directors.
Compensation Arrangements of the Chairman of the Board of Directors
| COMPENSATION ARRANGEMENTS OF THE CHAIRMAN OF THE BOARD OF DIRECTORS |
On December 31, 1998, we entered into an employment agreement with the Chairman of the board of directors, Dr. Vagelos. Dr. Vagelos did not become an officer of the Company or change his title. Pursuant to the terms of his employment agreement, Dr. Vagelos receives an annual salary of $100,000. In$100,000 as a non-officer employee.
Under the employment agreement, we agreed to recommend to theCurrent Compensation Committee that stock option grants be made to Dr. Vagelos for calendar years 2000 through 2003 in the amount of the greater of (a) 125,000 shares or (b) 125% of the highest annual option award granted to an officer of the Company.
In 2011, the Compensation Committee determined that Dr. Vagelos's target grant would be equal to ten times the annual grant for a non-employee member ofProgram, the board of directors setting his target award at 150,000 sharesdetermined that stock options and performance-based restricted stock units (also referred to as “PSUs”) would comprise 70% and 30% of common stock underlying stock options. In December 2013, 2014, and 2015, the Compensation Committee reduced the2019 annual equity award to Dr. Vagelos, by 15% each time,respectively, and set the targeted aggregate grant date fair value of such award at (but no more than) ten times the aggregate grant date fair value of the corresponding annual equity award for our non-employee directors. As in lineprior years, Dr. Vagelos’s 2019 annual equity award reflects, among other things, the key contributions that Dr. Vagelos makes as the Company continues to develop into a fully integrated biotech company with multiple class-leading products, as well as Dr. Vagelos’s crucial role as a trusted advisor to the reductionCEO, other senior managers, and the non-employee directors. It is also designed to incentivize further contributions and ensure Dr. Vagelos’s continued service to the Company in the annual stock option awards to our executive officers and the reduction in the annual stock option awards to the non-employee directors made in January 2014, 2015, and 2016. On December 16, 2015, the Compensation Committee granted Dr. Vagelos stock options to purchase 92,123 shares of common stock, at an exercise price of $555.67 per share, the fair market value per share of our common stock on the date of grant (determined as the average of the high and low sales price per share of common stock on the NASDAQ Global Select Market on the date of grant). future.
The 2019 stock option award granted to Dr. Vagelos vests ratably over four years subject to his continued service and contains change-of-control provisions consistent with those described above for stock optionequity grants to non-employee directors. The 2019 PSU award granted to Dr. Vagelos contains vesting conditions consistent with those described below under “Compensation Discussion and Analysis – Components of Executive Pay: What We Pay and Why We Pay It – Annual Equity Awards.” In addition, if any PSUs held by Dr. Vagelos vest upon a change of control (as a result of performance exceeding the relevant TSR goal for the period from the date of grant to the date of the change of
30 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| BOARD OF DIRECTORS / COMPENSATION OF DIRECTORS |
control), the earned shares will be delivered within 30 days of the vesting determination. Pursuant to the terms of his employment agreement, if Dr. Vagelos dies or is disabled during the term of his employment, all stock options granted to him by the Company will immediately become vested and exercisable.
21 In addition, the award agreements with Dr. Vagelos relating to his 2018 and 2019 stock option awards and 2019 PSU award provide that each such award will continue to vest in accordance with the terms of the applicable award agreement following his qualified retirement (as defined in the applicable Company policy; Dr. Vagelos currently meets the policy requirement).
Corporate Governance
The following table and explanatory footnotes provide information with respect to compensation paid to Dr. Vagelos and each non-employee director for their service in 2015
2019 in accordance with the policies, plans, and employment agreement described above:
Director Compensation
| A | B | C | D | E | F | G | H | |||||||||||||||||
| Name | Fees earned or paid in cash ($) | Stock awards1 ($) | Option awards1,2 ($) | Non-equity incentive plan compensation ($) | Change in pension value and non-qualified deferred compensation earnings | All other compensation3 ($) | Total ($) | |||||||||||||||||
| Bonnie L. Bassler, Ph.D. | 110,000 | 119,962 | 480,031 | — | — | — | 709,993 | |||||||||||||||||
| Michael S. Brown, M.D. | 120,000 | 119,962 | 480,031 | — | — | 5,000 | 4 | 724,993 | ||||||||||||||||
| N. Anthony Coles, M.D. | 100,000 | 119,962 | 480,031 | — | — | 5,000 | 4 | 704,993 | ||||||||||||||||
| Joseph L. Goldstein, M.D. | 110,000 | 119,962 | 480,031 | — | — | — | 709,993 | |||||||||||||||||
| Christine A. Poon | 120,000 | 119,962 | 480,031 | — | — | — | 719,993 | |||||||||||||||||
| Arthur F. Ryan | 120,000 | 119,962 | 480,031 | — | — | 5,000 | 4 | 724,993 | ||||||||||||||||
| George L. Sing | 120,000 | 119,962 | 480,031 | — | — | 3,000 | 4 | 722,993 | ||||||||||||||||
| Marc Tessier-Lavigne, Ph.D. | 100,000 | 119,962 | 480,031 | — | — | — | 699,993 | |||||||||||||||||
| P. Roy Vagelos, M.D. | — | 1,799,9445 | 4,199,881 | — | — | 105,000 | 6 | 6,104,825 | ||||||||||||||||
| Huda Y. Zoghbi, M.D. | 110,000 | 119,962 | 480,031 | — | — | 5,000 | 4 | 714,993 | ||||||||||||||||
| 1 | The amounts in columns (c) and (d) reflect the respective aggregate grant date fair values of RSUs or PSUs (as applicable) and options awarded during the year ended December 31, 2019 pursuant to the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Valuation assumptions and methodologies used in the calculation of these amounts do not take into account expected forfeitures and are otherwise described in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2019 included in the 2019 Annual Report. |
| 2 | At December 31, 2019, the non-employee directors and Dr. Vagelos had the following aggregate number of stock option awards outstanding: Dr. Bassler: 29,014; Dr. Brown: 41,353; Dr. Coles: 23,743; Dr. Goldstein: 41,103; Ms. Poon: 121,383; Mr. Ryan: 52,603; Mr. Sing: 111,853; Dr. Tessier-Lavigne: 81,132; Dr. Vagelos: 1,258,645; and Dr. Zoghbi: 29,014. |
| 3 | See the subsection “Compensation-Related Matters—Compensation Dashboard—Additional Compensation Information—Perquisites and Personal Benefits” for information regarding director air transportation in accordance with guidelines approved by our board of directors. |
| 4 | Consists of a Company contribution paid or payable on or before April 14, 2020 under the Regeneron Matching Gift Program in respect of charitable gifts made in 2019. |
| 5 | Consists of a PSU award with possible minimum, threshold, and maximum payouts of 2,019 shares, 4,038 shares, and 9,086 shares of common stock, respectively. |
| 6 | Consists of (i) $100,000 for the salary paid pursuant to the terms of our employment agreement with Dr. Vagelos and (ii) $5,000 for 401(k) Savings Plan matching contributions in respect of 2019. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  /31 /31 |
Name | | Fees earned or paid in cash ($) (b) | Stock awards ($) (c) | | Option awards ($) 1,2 (d) | Non-equity incentive plan compensation ($) (e) | Change in pension value and non-qualified deferred compensation earnings (f) | | All other compensation ($) (g) | | Total ($) (h) | ||||||||
Charles A. Baker | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Michael S. Brown, M.D. | | 75,000 | – | | 1,986,604 | – | – | | 5,000 | 3 | | 2,066,604 | |||||||
Alfred G. Gilman, M.D., Ph.D.4 | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Joseph L. Goldstein, M.D. | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Robert A. Ingram5 | | 65,000 | | | 1,986,604 | – | – | | – | | 2,051,604 | ||||||||
Christine A. Poon | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
Arthur F. Ryan | | 75,000 | – | | 1,986,604 | – | – | | 2,500 | 3 | | 2,064,104 | |||||||
George L. Sing | | 80,000 | – | | 1,986,604 | – | – | | 5,000 | 3 | | 2,071,604 | |||||||
Marc Tessier-Lavigne, Ph.D. | | 75,000 | – | | 1,986,604 | – | – | | – | | 2,061,604 | ||||||||
P. Roy Vagelos, M.D. | | – | – | | 23,098,558 | – | – | | 109,000 | 6 | | 23,207,558 | |||||||
| | | | | | | | | | | | | | | | | | | | |
01
The
amounts in column (d) reflectboard of directors, upon theaggregate grant date fair valuerecommendation ofoptions awarded duringtheyear ended December 31, 2015 pursuant toCorporate Governance and Compliance Committee, has nominated for election at theRegeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Assumptions used in2020 Annual Meeting Joseph L. Goldstein, M.D., Christine A. Poon, P. Roy Vagelos, M.D., and Huda Y. Zoghbi, M.D. as Class II Directors for a three-year term expiring at thecalculation of this amount do not take into account expected forfeitures2023 Annual Meeting andare otherwise described in Note 14 to the Company's audited financial statements for the fiscal year ended December 31, 2015 included in the 2015 Annual Report.2At December 31, 2015, the non-employee directors and Dr. Vagelos had the following stock option awards outstanding: Mr. Baker: 88,588; Dr. Brown: 35,588; Dr. Gilman: 38,588; Dr. Goldstein: 43,588; Mr. Ingram: 0; Ms. Poon: 93,118; Mr. Ryan: 24,338; Mr. Sing: 105,588; Dr. Tessier-Lavigne: 52,867; and Dr. Vagelos: 2,270,363.
3Consists of a Company contribution paid or payable on or before April 14, 2016 under the Regeneron Matching Gift Program in respect of a charitable gift made in 2015.
4Dr. Gilman servedN. Anthony Coles, M.D. as adirector until his death on December 23, 2015.
5Mr. Ingram served as a director until his resignation on November 10, 2015.
6Consists of (i) $100,000 for the salary paid pursuant to the terms of our employment agreement with Dr. Vagelos, (ii) $4,000 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016, and (iii) $5,000Class III Director for aCompany contribution paid or payable on or before April 14, 2016 underone-year term expiring at theRegeneron Matching Gift Program in respect of a charitable gift made in 2015.
22Corporate Governance
Executive Officers of the Company2021 Annual Meeting.The board of directors unanimously recommends a voteFORthe election of each of these nominees.
32 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING 
EXECUTIVE OFFICERS OF THE COMPANY
All officers of the Company are appointed annually and serve at the pleasure of the board of directors. The names, positions, ages, and background of the
Company'sCompany’s executive officers as of April 14,20162020 are set forth below.Except as identified below, thereThere are no family relationships between any of our directors and executive officers. None of the corporations or other organizations referred to below with which an executive officer has previously been employed or otherwise associated is a parent, subsidiary, or affiliate of the Company.LEONARD S. SCHLEIFER, M.D., Ph.D.,63, co-founded
Leonard S. Schleifer, M.D., Ph.D.,67, founded the Company in 1988, has been a Director and its President and Chief Executive Officer since its inception, and served as Chairman of the Board from 1990 through 1994. Dr. Schleifer, together with Regeneron’s founding scientist, Dr. Yancopoulos, built and has managed the Company over the past 32 years. Dr. Schleifer received his M.D. and Ph.D. in Pharmacology from the University of Virginia. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology. 
George D. Yancopoulos, M.D., Ph.D.,60, joined Dr. Schleifer in 1989 as founding scientist of the Company, and together they built and have managed the Company since then. Dr. Yancopoulos is currently President and Chief Scientific Officer, and has served on the board since 2001. He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and/or developer of the seven FDA-approved drugs the Company has developed, EYLEA®, Praluent®, Dupixent®, Kevzara®, Libtayo®, ZALTRAP®, and ARCALYST®, as well as of its foundation technologies, including the TRAP technology,VelociGene®, andVelocImmune®. 
Christopher Fenimore,49, has been Vice President, Controller since March 2017. From January 2017 to March 2017, he served as Vice President, Deputy Controller, and previously served as Vice President, Financial Planning from January 2012 to December 2016. Prior to joining the Company in 2003, he was Vice President, Finance at Mojave Therapeutics, Inc. Mr. Fenimore’s prior experience includes working as a supervising senior accountant at KPMG, as well as healthcare industry- focused venture capital and investment banking roles. Mr. Fenimore holds an M.A. in Biotechnology from Columbia University, an M.B.A. in Professional Accounting from Rutgers Business School, and a B.A. in Economics from Rutgers University. Mr. Fenimore is a Certified Public Accountant in the State of New York. 
Robert E. Landry,56, has been Executive Vice President, Finance since January 2019 and Chief Financial Officer since October 2013. From September 2013 to December 2018, he served as Senior Vice President, Finance. Previously, Mr. Landry served as Senior Vice President, Treasurer, at Pfizer Inc. from October 2012 to August 2013 and Senior Vice President—Finance, Pfizer’s Diversified Business, from October 2009 to October 2012. Prior to those roles, Mr. Landry held a number of positions at Wyeth, which was acquired by Pfizer Inc. in October 2009, including Treasurer and Principal Corporate Officer from 2007 to 2009, Director of Pharmaceutical Marketing and Sales of Wyeth’s Australian affiliate from 2006 to 2007, and Chief Financial Officer of Wyeth’s Australian and New Zealand affiliates from 2004 to 2006. 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  / 33
/ 33THE COMPANY / EXECUTIVE OFFICERS OF THE COMPANY

Joseph J. LaRosa,61, has been Executive Vice President, General Counsel and Secretary since January 2019. From September 2011 to December 2018, he served as Senior Vice President, General Counsel and Secretary. Before joining Regeneron, Mr. LaRosa was Senior Vice President, General Counsel, and Secretary at Nycomed US Inc. Mr. LaRosa’s prior experience includes working in a number of senior legal positions at Schering-Plough Corporation from 1993 to 2009, where he was a corporate officer and served most recently as Vice President, Legal Affairs, and a member of the Operations Management Team. Mr. LaRosa received his J.D. from New York University School of Law. 
Marion McCourt,60, has been Senior Vice President, Commercial since February 2018. From April 2017 until joining the Company, Ms. McCourt served as the Principal Operating Officer and the Chief Operating Officer and President of Axovant Sciences, Inc. Ms. McCourt previously served as chief operating officer of Medivation, Inc. from February 2016 until its acquisition by Pfizer Inc. in September 2016. Previously, Ms. McCourt worked at Amgen Inc., where she most recently served as a Vice President in U.S. Commercial Operations from February 2014 to January 2016. From May 2013 to January 2014, Ms. McCourt served as Vice President and General Manager at Amgen where she was responsible for the bone health and primary care business unit. From 2012 to 2013, she was Chief Operating Officer for AstraZeneca U.S., a division of AstraZeneca plc. Her responsibilities included oversight and leadership of all U.S. commercial functions, including medical affairs, business development, finance, human resources, legal, operations, and corporate affairs. During her 12-year tenure at AstraZeneca, Ms. McCourt was President and Chief Executive Officer of AstraZeneca Canada Inc. from 2011 to 2012 and also held various other roles at AstraZeneca Pharmaceuticals LP, a subsidiary of AstraZeneca plc. Ms. McCourt received her B.S. in Biology from Lafayette College. 
Andrew J. Murphy, Ph.D.,62, has been Executive Vice President, Research since January 2019. He previously served as Senior Vice President, Research, Regeneron Laboratories from January 2013 to December 2018, as Vice President, Target Discovery from May 2005 to December 2012, as Vice President, Gene Discovery and Bioinformatics from January 2001 to May 2005, and Director of Genomics and Bioinformatics from May 1999 to December 2000. Dr. Murphy is a co-inventor of several of the Company’s key technologies, includingVelociGene®andVelocImmune®, and continues to lead several technology centers and therapeutic focus areas. He received his B.S. in Molecular Biology at the University of Wisconsin, and his Ph.D. in Human Genetics from Columbia University, College of Physicians and Surgeons. 
Neil Stahl, Ph.D.,63, has been Executive Vice President, Research and Development since January 2015. He previously served as Senior Vice President, Research and Development Sciences from January 2007 to December 2014, as Senior Vice President, Preclinical Development and Biomolecular Sciences from December 2000 to December 2007, and as Vice President, Preclinical Development and Biomolecular Sciences from January 2000 to December 2000. He joined the Company in 1991. Before becoming Vice President, Biomolecular Sciences in 1997, Dr. Stahl was Director, Cytokines and Signal Transduction. Dr. Stahl received his Ph.D. in Biochemistry from Brandeis University. 
Daniel P. Van Plew,47, has been Executive Vice President and General Manager, Industrial Operations and Product Supply since January 2016. From April 2008 to December 2015, Mr. Van Plew served as Senior Vice President and General Manager, Industrial Operations and Product Supply. Prior to that date, he served as Vice President and General Manager, Industrial Operations and Product Supply since joining the Company in 2007. From 2006 until 2007, Mr. Van Plew served as Executive Vice President, R&D and Technical Operations of Crucell Holland B.V., a global biopharmaceutical company. Between 2004 and 2006, Mr. Van Plew held positions of increasing responsibility at Chiron Biopharmaceuticals, part of Chiron Corporation, a biotechnology company, most recently as Senior Director, Vacaville Operations. From 1998 until 2004, Mr. Van Plew held various managerial positions in the health and life sciences practice at Accenture, Ltd., a management consulting business. Mr. Van Plew received his M.S. in Chemistry from The Pennsylvania State University and his M.B.A. from Michigan State University. 34 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING 
Regeneron is committed to good corporate governance, which we believe promotes the long-term interests of shareholders, strengthens the accountability of the
Board from 1990 through 1994. Dr. Schleifer received his M.D. and Ph.D. in Pharmacology from the University of Virginia. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology.GEORGE D. YANCOPOULOS, M.D., Ph.D.,56, joined the Company in 1989 as its Founding Scientist and is currently President, Regeneron Laboratories and Chief Scientific Officer. While holding leadership positions, Dr. Yancopoulos headed the Company's laboratories and science organization since joining the Company and, in 1998, was named the Company's first Chief Scientific Officer. Dr. Yancopoulos joined theboardin 2001. He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and developer of the four FDA-approved drugs the Company has developed, EYLEA®, Praluent®, ZALTRAP®, and ARCALYST®, as well as of its foundation technologies, including the TRAP technology,VelociGene®, andVelocImmune®.MICHAEL ABERMAN, M.D.,45, has been Senior Vice President, Strategy and Investor Relations since January 2015. From March 2010 to December 2014, he served as Vice President, Strategy and Investor Relations. Prior to joining the Company, he spent six years as a Wall Street analyst covering the biotechnology industry. From March 2006 until joining the Company, he was Director and Senior Biotechnology Analyst at Credit Suisse. Prior to that, from March 2004 until March 2006, he worked as a Biotechnology Analyst at Morgan Stanley, Inc. From February 2002 through March 2004, Dr. Aberman was Director of Business Development at Antigenics Inc., an oncology-focused biotechnology company. Dr. Aberman received his M.D. with honors from the University of Toronto and his M.B.A. from the Wharton School of the University of Pennsylvania.ROBERT E. LANDRY,52, has been Senior Vice President, Finance since September 2013 and Chief Financial Officer since October 2013. Previously, Mr. Landry served as Senior Vice President, Treasurer, at Pfizer Inc. from October 2012 to August 2013 and Senior Vice President – Finance, Pfizer's Diversified Business, from October 2009 to October 2012. Prior to those roles, Mr. Landry held a number of positions at Wyeth, which was acquired by Pfizer Inc. in October 2009, including Treasurer and Principal Corporate Officer from 2007 to 2009, Director of Pharmaceutical Marketing and Sales of Wyeth's Australian affiliate from 2006 to 2007, and Chief Financial Officer of Wyeth's Australian and New Zealand affiliates from 2004 to 2006.JOSEPH J. LAROSA,57, has been Senior Vice President, General Counsel, and Secretary since September 2011. Before joining Regeneron, Mr. LaRosa was Senior Vice President, General Counsel, and Secretary at Nycomed US Inc. Mr. LaRosa's prior experience includes working in a number of senior legal positions at Schering-Plough Corporation from 1993 to 2009, where he was a corporate officer and served most recently as Vice President, Legal Affairs, and a member of the Operations Management Team. Mr. LaRosa received his J.D. from New York University School of Law.DOUGLAS S. McCORKLE,59, has been Vice President, Controller, and Assistant Treasurer since 2007. Prior to that date, he served as Controller and Assistant Treasurer since 1998. Prior to joining the Company, Mr. McCorkle was Controller of Intergen Company, a manufacturer of biopharmaceutical products, a position he held since 1997. From 1990 to 1996, Mr. McCorkle was employed with Coopers & Lybrand L.L.P., where he specialized in biotechnology clients and served in various positions including Audit Manager from 1995 to 1996. As previously reported, Mr. McCorkle has notified the Company that he plans to retire in early 2017.PETER POWCHIK, M.D.,59, has been Senior Vice President, Clinical Development since joining the Company in October 2006. Prior to joining the Company, Dr. Powchik was employed at several pharmaceutical companies, serving as Senior Vice President and Chief Medical Officer of Chugai Pharma USA, a position he held from May 2005 until October 2006. From April 2001 until May 2005, he held various senior clinical development positions at Novartis Pharmaceuticals Corporation, most recently as Vice President, US Clinical Development and Medical Affairs. Dr. Powchik held various clinical development positions with Sepracor Inc. and Pfizer Inc. from October 1996 to April 2001.23Executive Officers of the CompanyDr. Powchik received his M.D. from New York University School of Medicine.NEIL STAHL, Ph.D.,59, has been Executive Vice President, Research and Development since January 2015. He previously served as Senior Vice President, Research and Development Sciences from January 2007 to December 2014, as Senior Vice President, Preclinical Development and Biomolecular Sciences from December 2000 to December 2007, and as Vice President, Preclinical Development and Biomolecular Sciences from January 2000 to December 2000. He joined the Company in 1991. Before becoming Vice President, Biomolecular Sciences in July 1997, Dr. Stahl was Director, Cytokines and Signal Transduction. Dr. Stahl received his Ph.D. in Biochemistry from Brandeis University.ROBERT J. TERIFAY,56, has been Executive Vice President, Commercial since January 2016. From February 2007 to December 2015, he served as Senior Vice President, Commercial. Prior to joining the Company, Mr. Terifay was employed at several biopharmaceutical companies. From January to October 2006, Mr. Terifay served as President and Chief Operating Officer of Arginox Pharmaceuticals. Prior to his employment at Arginox, Mr. Terifay was Senior Vice President, Business Operations at Synta Pharmaceuticals from March to December 2005. From February 2002 until March 2005, he held various senior commercial and marketing positions at Millennium Pharmaceuticals, Inc., most recently as Senior Vice President, Oncology Commercial. Mr. Terifay was Vice President, Marketing at Cor Therapeutics, Inc. from 1996until its acquisition by Millennium Pharmaceuticals, Inc. in February 2002. Mr. Terifay was Executive Vice President of Strategic Services at Saatchi & Saatchi, an advertising firm, from 1993 to 1996. From 1985 to 1993, he held various commercial and marketing positions at G.D. Searle & Company. Mr. Terifay received his Master of Management degree in Marketing and Health Service Management from the J.L. Kellogg Graduate School of Management, Northwestern University.DANIEL P. VAN PLEW,43, has been Executive Vice President and General Manager, Industrial Operations and Product Supply since January 2016. From April 2008 to December 2015, Mr. Van Plew served as Senior Vice President and General Manager, Industrial Operations and Product Supply. Prior to that date, he served as Vice President and General Manager, Industrial Operations and Product Supply since joining the Company in 2007. From 2006 until 2007, Mr. Van Plew served as Executive Vice President, R&D and Technical Operations of Crucell Holland B.V., a global biopharmaceutical company. Between 2004 and 2006, Mr. Van Plew held positions of increasing responsibility at Chiron Biopharmaceuticals, part of Chiron Corporation, a biotechnology company, most recently as Senior Director, Vacaville Operations. From 1998 until 2004, Mr. Van Plew held various managerial positions in the health and life sciences practice at Accenture, Ltd., a management consulting business. Mr. Van Plew received his M.S. in Chemistry from The Pennsylvania State University and his M.B.A. from Michigan State University.24Executive Officers of the Company
Security Ownership of Certain Beneficial Owners and ManagementThe following table sets forth, as of April 14, 2016, the number of shares of the Company's Class A stock and common stock beneficially owned by each of the Company's directors, each of the Named Officers referred to below under "Executive Compensation," all directors and executive officers as a group, and each other person or group of persons known by the Company to beneficially own more than 5% of the outstanding shares of common stock or Class A stock, based upon (unless indicated otherwise) information obtained from such persons, and the percentage that such shares represent of the number of outstanding shares of Class A stock and common stock, respectively.The Class A stock is convertible on a share-for-share basis into common stock. The Class A stock is entitled to ten votes per share and the common stock is entitled to one vote per share. We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, we believe, based on the information furnished or otherwise available to us, that the persons named in the table below have sole voting and investment power with respect to all shares of Class A stock and common stock shown as beneficially owned by them, subject to applicable community property laws. We have based our calculation of percentage of shares of a class beneficially owned on1,913,136 shares of Class A stock and 103,165,457 shares of common stock outstanding as of April 14, 2016, except that for each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock beneficially owned by that person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person assume the conversion on April 14, 2016 of all shares of Class A stock listed as beneficially owned by such person (or persons in the caseof directors and management, and helps build trust in the Company. The following chart summarizes key information regarding our corporate governance.Board and Other Governance Information 2020* Size of Board 12 Number of Independent Directors 9 Separate Chairman and Chief Executive Officer 
Majority Voting in the Election of Directors 
Director Resignation Policy 
Number of Meetings of the Board of Directors Held in 2019 6 Independent Directors Meet in Executive Sessions Without Management Present 
Code of Business Conduct and Ethics Applicable to All Employees, Officers, and Directors 
Annual Board and Committee Self-Evaluations 
Stock Ownership Guidelines for Directors and Senior Executives 
Active Shareholder Engagement 
Shareholder Right to Call Special Shareholder Meeting 
* As of April 14, 2020. The board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers, and directors. You can find links to this code on our website atwww.regeneron.comunder the “Corporate Governance” heading on the “Investors & Media” page. We may satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or a waiver from, a provision of our code of business conduct and ethics that applies to our principal executive
officers asofficer, principal financial officer, principal accounting officer, or controller, or persons performing similar functions, by posting such information on our website where it is accessible through the same link noted above.SUCCESSION PLANNING AND TALENT DEVELOPMENT PROCESS
Under our Corporate Governance Guidelines, the board of directors is required to periodically review with our CEO Regeneron’s plan for succession to the offices of the CEO and other senior executive positions. In 2017, the Corporate Governance and Compliance Committee, at the request of the board of directors, commenced a
group) into common stockmulti-year formal succession planning andalso that notalent review, which includes succession planning for the CEO and othersharessenior management positions. In 2018 and 2019, the applicable committees ofClass A stock beneficially ownedthe board of directors advanced this formal review byothers are so converted.focusing on certain assigned functions and roles within the Company. As part of this process, the Audit Committee reviewed functions and roles within the Company’s finance, information technology, and real estate & facilities management organizations, while the Compensation Committee and the Technology Committee reviewed functions and roles within the Company’s commercial organization and the Company’s research & development and global development organizations, respectively. Implementation of the succession planning and talent review plan has continued in 2020.2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  / 35
/ 35THE COMPANY / CORPORATE GOVERNANCE
In
computingaddition to formal succession planning, directors also have exposure to Regeneron leaders through board and committee presentations and discussions and informal events and interactions with key talent throughout thenumberyear, both in small-group and one-on-one settings.Regeneron’s mission is to use the power of
shares of common stock beneficially ownedscience to repeatedly bring new medicines to patients. We are committed to operating responsibly, communicating transparently about our impacts, and engaging all stakeholders in our mission. We strive to “do well bya person (and by directorsdoing good” andexecutive officers as a group) and the percentage ownership of common stock of such person (and by directors and executive officers as a group), shares of common stock subject to options held by that person (and by directors and executive officers as a group) that are exercisable as of April 14, 2016 or are exercisable within sixty days after April 14, 2016 are deemed to be outstanding. Such shares are not deemed to be outstanding, however, for the purpose of computing the percentage ownership of common stock of any other person.25have been publicly disclosing information about significant corporate responsibility matters since 2014.Security Ownership of Certain Beneficial Owners and Management
Shares of Class A Stock
Beneficially Owned1
Shares of Common Stock
Beneficially Owned1 Name and Address of Beneficial Owner
Number Percent
of Class Number2 Percent
of ClassBeneficial Owners of 5% or More of Common Stock or
Class A Stock (Other Than Directors and Executive Officers): Sanofi3
— — 23,353,665 22.6% 54, rue La Boetie
75008 Paris
France
Capital World Investors4
— — 6,804,465 6.6% 333 South Hope Street
Los Angeles, California 90071
FMR LLC5
— — 6,223,092 6.0% 245 Summer Street
Boston, Massachusetts 02210
BlackRock, Inc.6
— — 5,382,473 5.2% 55 East 52nd Street
New York, New York 10055
Directors and Executive Officers:7
Leonard S. Schleifer, M.D., Ph.D.
1,726,565 8 90.3% 3,993,844 9 3.7% P. Roy Vagelos, M.D.
— — 2,875,335 10 2.7% George D. Yancopoulos, M.D., Ph.D.
42,750 11 2.2% 2,967,079 12 2.8% Charles A. Baker
62,384 13 3.3% 148,497 14 * Michael S. Brown, M.D.
— — 47,462 15 * Joseph L. Goldstein, M.D.
— — 40,113 16 * Christine A. Poon
— — 82,433 17 * Arthur F. Ryan
— — 53,363 18 * George L. Sing
— — 224,385 19 * Marc Tessier-Lavigne, Ph.D.
— — 42,579 20 * Robert E. Landry
— — 41,656 21 * Neil Stahl, Ph.D.
— — 405,164 22 * Robert J. Terifay
— — 197,466 23 * All Directors and Executive Officers as a Group (18 persons)
1,831,699 95.7% 11,720,333 24 10.4% *Represents less than 1%
1The inclusion herein of any Class A stock or common stock, as the case may be, deemed beneficially owned does not constitute an admission of beneficial ownership of those shares.
2For each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock listed includes the number of shares of Class A stock listed as beneficially owned by such person (or persons in the caseOur board of directors andexecutive officers asmanagement team focus closely on corporate responsibility matters. The charter of the board’s Corporate Governance and Compliance Committee expressly delegates board oversight of corporate responsibility to the Committee. The Company’s policy is to take into consideration the long-term interests of the Company, its shareholders, and other stakeholders, including patients, employees, the healthcare community, regulators, partners, suppliers, and local communities. Under our Corporate Governance Guidelines, the Corporate Governance and Compliance Committee is responsible for overseeing the Company’s key corporate responsibility initiatives, including those expected to have agroup).
3Based solelysignificant impact on the Company’s ability to deliver sustained growth. The Committee also conducts aForm 4 filed by Sanofiperiodic review of environmental, social, and governance (“ESG”) matters. Management, who has the responsibility for formulating and implementing such initiatives and matters, has established for these purposes a Responsibility Committee comprised of cross-functional business leaders.Our responsibility strategy centers on three focus areas:
• Improve the lives of people with serious diseases • Foster a culture of integrity and excellence • Build sustainable communities In 2019, we set global 2025 responsibility goals, which span across these three focus areas and the environmental and social issues that we believe are most significant to our business and stakeholders. These goals, summarized below, are discussed in detail in our 2019 Responsibility Report.
36 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING
THE COMPANY / CORPORATE GOVERNANCE 2025 GLOBAL RESPONSIBILITY GOALS
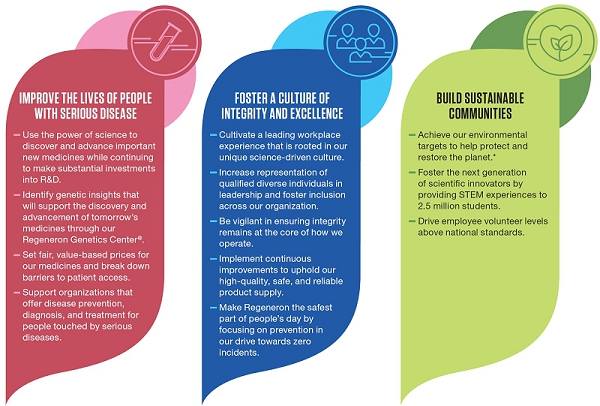
* See the chart below for our specific environmental targets.
In 2019, we also announced our support of the United Nations Sustainable Development Goals and identified five of such goals where we intend to focus to deliver the most impact: good health and well-being; quality education; gender equality; responsible consumption and production; and partnerships for the goals. Our support of these goals helped guide our selection of the 2025 responsibility goals listed above.
We are proud to have been added to the Dow Jones Sustainability World Index (“DJSI World”) for the first time in 2019, representing one of only four companies from the biotechnology sector recognized. DJSI World is a leading global index comprised of corporate leaders in ESG practices, which recognizes the top 10 percent most sustainable companies in each industry.
In 2019 and 2020 to date, we have also continued to enhance our reporting of the Company’s responsibility efforts and results. For the first time, our 2019 Responsibility Report aligned our disclosures with the
SECSustainability Accounting Standards Board (“SASB”) framework. In addition, we are working toward aligning our reporting for the 2020 Responsibility Report with the climate-specific recommendations developed by the Task Force onFebruary 18, 2016. AccordingClimate-related Financial Disclosures (“TCFD”).Improve the lives of people with serious diseases.As a science-focused company, we operate Regeneron with the long-term outlook required to turn rigorous scientific research into important new medicines. All seven of our approved medicines and the product candidates in our clinical pipeline are homegrown – discovered in Regeneron’s labs using our industry-leading, proprietary technologies. Our support for patients extends beyond the labs to disease education and awareness efforts, product support services, and our commitment to drug access and responsible pricing.
2019 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  /37
/37THE COMPANY / CORPORATE GOVERNANCE Foster a culture of integrity and excellence.Regeneron’s unique culture makes us who we are. Our culture includes our science-led mindset, our high ethical standards, and our unbridled focus on solving big, complex problems. As we continue to grow, we remain committed to making significant investments to attract and retain top talent and facilitate the diverse and inclusive workforce we require to bring new medicines to people in need. At the end of 2019, 49% of our full-time employees, and 39% of those in leadership positions, were women.
We are equally committed to conducting our business responsibly and ethically. This is demonstrated through the range of policies, practices, and initiatives we have implemented, encompassing areas such as compliance, responsible sales and marketing, ethical clinical trials, responsible supply chain, and product quality and safety.
Build sustainable communities.We believe that our role in creating a healthier world extends beyond creating life-transforming medicines to building a healthy living environment. In 2019, in connection with the selection of our 2025 responsibility goals, we set ambitious medium- and longer-term environmental sustainability targets, as shown below. We plan to begin reporting on our progress toward these goals in the 2020 Responsibility Report.
New Environmental Targets to Help Protect and Restore the Planet

By 2025, improve water efficiencies by implementing global water mapping strategy and water stewardship program. 
By 2021, achieve zero waste to landfill status at all Regeneron sites.*
By 2021, compost food waste at all sites with more than 2,000 employees.
By 2025, develop and implement waste management plans to further increase our plastic recycling and reduce hazardous waste generation. 
By 2021, engage our top 30 suppliers, representing more than 50% of spend, to gather and report relevant scope 3 greenhouse gas (GHG) emissions data. By 2023, set global sciencebased targets for scope 1 and 2 GHG emissions. By 2025, match 50% of our electricity consumption with electricity from certified renewable energy sources.
By 2025, invest in the production of renewable power to meet our long-term electricity needs.
By 2025, reduce combined scope 1 & 2 (market-based) GHG emissions per square meter by 30% based on 2016 peak baseline.
By 2035, match 100% of our electricity consumption with electricity from certified renewable energy sources.
2021 2023 2025 2035 *Excludes construction and demolition waste
We also strengthen our communities through strategic philanthropic investments, product donations, and the power of our employees’ talents and time. We are a long-standing supporter of science, technology, engineering and math (“STEM”) education, and make major philanthropic investments to inspire and celebrate future scientific innovators, including our ten-year, $100-million commitment to the Regeneron Science Talent Search, the nation’s most prestigious pre-college science and mathematics competition; and our new five-year, $24-million commitment to the Regeneron International Science and Engineering Fair, the world’s largest pre-college science and engineering competition. We also launched the Regeneron DNA Learning Center, a program of Cold Spring Harbor Laboratory, in 2019. Equipped with two state-of-the-art teaching labs, this
Form 4, 20,554,113interactive educational center hosts local middle- and high-school field trips during the academic year, summer camps, and extended research projects. Overall, STEM education represents approximately 86% of our corporate philanthropy grants made in 2019, not including medical grants and matched funds.38 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING THE COMPANY / CORPORATE GOVERNANCE In 2019, 59% of Regeneron worldwide employees donated more than 27,800 hours to local non-profit organizations through our volunteer programs. This is in the top-quartile of corporate volunteer participation rates, and well above the 33% corporate average rate, according to a benchmarking study published in 2019 byChief Executives for Corporate Purpose. We also held our third annual Day for Doing Good, a company-wide day of service that had 57% employee participation — a record high for the Company. We are proud to be recognized for the third consecutive year as one of the
shares are held directly50 most community-minded companies in the United States bysanofi-aventis Amerique du Nordthe non-profit organization Points of Light.For more information about our responsibility efforts and
2,799,552 of the shares are held directly by Aventis Pharmaceuticals Inc. sanofi-aventis Amerique du Nord is a direct, wholly-owned subsidiary of Sanofi, and Aventis Pharmaceuticals Inc. is an indirect, wholly-owned subsidiary of sanofi-aventis Amerique du Nord. Pursuantresults, please refer to theAmended and Restated Investor Agreement, dated as of January 11, 2014, by and among Sanofi, sanofi-aventis US LLC, Aventis Pharmaceuticals Inc., and sanofi-aventis Amerique du Nord (collectively,2019 Responsibility Report available on our website.We are committed to adhering to the
"Sanofi Parties"), andhighest ethical standards when engaging in any political activities. Reflecting this commitment, theCompany, the Sanofi Parties have agreed to vote all shares of our voting securities they are entitled to vote from time to time as recommended by ourboard of directorsexcept that they may elect to vote proportionally with(upon thevotes cast by all of our other shareholders with respect to certain change-of-control transactions and to vote in their sole discretion with respect to liquidation or dissolution of Regeneron, stock issuances equal to or exceeding 20%recommendation of thethen outstanding shares or voting rights of common stockCorporate Governance andClass A stock (taken together), and new equity compensation plans or amendments if not materially consistentCompliance Committee) has adopted the Company’s Corporate Political Contributions Policy, a formal written policy that, together with ourhistorical equity compensation practices. See "Certain Relationshipscode of business conduct andRelated Transactions – Transactions with Related Persons – Amendedethics, sets forth our policies andRestated Investor Agreement with Sanofi" for further information regarding the Amendedprocedures on political contributions andRestated Investor Agreement with Sanofi.26Security Ownership of Certain Beneficial Owners and Management4Based solelypolitical activity. The policy is available onan amendment to a Schedule 13G filed by Capital World Investors on February 16, 2016. According to this amendment, Capital World Investors, a division of Capital Research and Management Company, has sole voting and dispositive power as to all of the shares reported as beneficially owned. Capital World Investors is deemed to be the beneficial owner of such shares as a result of Capital Research and Management Company acting as investment adviser to various registered investment companies.
- our website at
5www.regeneron.com Based solely on an amendment to a Schedule 13G filed by FMR LLC on February 12, 2016. According to this amendment, FMR LLC has sole voting power as to 639,403 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. Abigail P. Johnson is a Director, the Vice Chairman, the Chief Executive Officer, and the President of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders' voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders' voting agreement, members of the Johnson family may be deemed to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies (the "Fidelity Funds") advised by Fidelity Management & Research Company, a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds' Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by the Fidelity Funds' Boards of Trustees.
6Based solely on an amendment to a Schedule 13G filed by BlackRock, Inc. on February 10, 2016. According to this amendment, BlackRock, Inc. has sole voting power as to 4,830,005, shared voting power as to 5,449, sole dispositive power as to 5,377,024, and shared dispositive power as to 5,449 of the shares reported as beneficially owned.
7The address for each director and executive officer is c/o Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707.
8Includes 15,775 shares of Class A stock held in trust for the benefit of Dr. Schleifer's son, of which Dr. Schleifer is a trustee.
9Includes (i) 2,218,771 shares of common stock purchasable upon the exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; and (ii) 5,702 shares of common stock held in an accountunder theCompany's 401(k) Savings Plan.
10Includes (i) 1,995,705 shares of common stock purchasable upon exercise of options granted pursuant to“Transparency & Policies” heading on theRegeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 2,290 shares of common stock held in an account under the Company's 401(k) Savings Plan; (iii) 152,964 shares of common stock held in a charitable lead annuity trust, of which Dr. Vagelos is the trustee; (iv) 92,947 shares of common stock held in a trust for his grandchildren, of which Dr. Vagelos's wife is the trustee; (v) 1,203 shares of common stock held in trusts for his grandchildren, of which Dr. Vagelos and/or his wife are trustees; and (vi) 56,159 shares of common stock and 241,187 shares of common stock held by the Marianthi Foundation and the Pindaros Foundation, respectively, both of which are charitable foundations of which Dr. Vagelos is a director and an officer. Dr. Vagelos disclaims beneficial ownership of the shares held by these charitable foundations.
11Of these shares, 23,367 shares are held in trust for the benefit of Dr. Yancopoulos's children and certain other family members; Dr. Yancopoulos is a trustee of the trust. The remaining 19,383 shares are held in custody for the benefit of Dr. Yancopoulos's children.
12Includes (i) 1,848,756 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 500,000 shares of restricted stock which vest on December 17, 2017; (iii) 5,676 shares of common stock held in an account under the Company's 401(k) Savings Plan; and (iv) 567,976 shares of common stock held in trust for the benefit of Dr. Yancopoulos's children and certain other family members, of which Dr. Yancopoulos is a trustee.
13All shares of Class A stock are held by a limited partnership, of which Mr. Baker is a general partner.
14Includes 77,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
15Includes (i) 24,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; and (ii) 6,000 shares of common stock held by a family charitable foundation of which Dr. Brown is a director and an officer and his wife is a director. Dr. Brown disclaims beneficial ownership of the shares held by this charitable foundation.
16Includes 27,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
17Includes 81,643 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
18Includes 12,863 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
2019 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  /39
/3927Security Ownership of Certain Beneficial Owners and Management19Includes (i) 94,113 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 3,000 shares of common stock held by Mr. Sing's spouse; (iii) 4,500 shares of common stock held by Mr. Sing's spouse as custodian for the benefit of their son; and (iv) 10,000 shares of common stock held in a trust for benefit of Mr. Sing's son.
20Includes 41,392 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016.
21Includes (i) 34,500 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 5,000 shares of restricted stock which vest on September 9, 2018; and (iii) 57 shares of common stock held in an account under the Company's 401(k) Savings Plan.
22Includes (i) 352,717 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 20,732 shares of common stock held in separate grantor retained annuity trusts of which Dr. Stahl is the trustee; and (iii) 5,621 shares of common stock held in an account under the Company's 401(k) Savings Plan.
23Includes (i) 172,500 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; and (ii) 1,673 shares of common stock held in an account under the Company's 401(k) Savings Plan.
24Includes (i) 7,483,044 shares of common stock purchasable upon exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan that are exercisable or become so within sixty days after April 14, 2016; (ii) 515,000 shares of unvested restricted stock; and (iii) 28,853 shares of common stock held in an account under the Company's 401(k) Savings Plan.
28Security Ownership of Certain Beneficial Owners and Management
Section 16(a) Beneficial Ownership Reporting ComplianceCERTAIN RELATIONSHIPS AND RELATED TRANSACTIONSBased solely upon a review of reports filed pursuant to Section 16(a) of the Exchange Act or written representations from reporting persons, the Company is not aware of any director, executive officer, or beneficial owner of more than 10%of our common stock who has not filed on a timely basis any report required by such Section 16(a) to be filed during or in respect of our fiscal year ended December 31, 2015.29Section 16(a) Beneficial Ownership Reporting Compliance
Certain Relationships and Related TransactionsREVIEW, APPROVAL, OR RATIFICATION OF TRANSACTIONS WITH RELATED PERSONS Review, Approval, or Ratification of Transactions with Related PersonsThe board of directors has adopted a written policy for the review, approval, or ratification of related person transactions. The Company considers transactions (or a series of related transactions) in which the Company is a participant, the amount involved exceeds $10,000 in any calendar year, and a director, officer, more than 5% holder of our voting securities, any immediate family member of any of the foregoing, or any related entity of any of the foregoing has a direct or indirect material interest to constitute related person transactions.
As amended in January 2015, theThe policy provides for a standing pre-approval of transactions with any passive institutional shareholder who holds more than 5% of our voting securities, transactions where all shareholders receive proportional benefits, and certain transactions with Sanofi. With respect to any new transaction that is deemed pre-approved, the Audit Committee receives a summary of each such transaction and retains the ability to require that one or more of such transactions be subject to the standard approval procedures. The policy also requires that the arrangements relating to a permanent, full-time employment of an immediate family member of a director or executive officer hired by the Company be approved in accordance with the policy. In addition, in the event a person is or becomes a director or executive officer of the Company and an immediate family member of such person is a permanent, full-time employee of the Company, no material, outside-of-the-ordinary-course-of-business change in the terms of employment, including compensation, are permitted to be made without the prior approval of the Audit Committee (except, if the immediate family member is himself or herself an executive officer of the Company, any proposed change in the terms of employment are reviewed and approved in the same manner as compensatory arrangements of other executive officers).The board of directors has determined that the members of the Audit Committee are best suited to review and approve related person transactions. Accordingly, each related person transaction (other than a transaction that is deemed pre-approved as described above) must be reviewed and approved or ratified by the members of the Audit Committee, other than any member of the Audit Committee that has an interest in the transaction. Under the policy, the Chairman of the Audit Committee is delegated the authority to approve certain related person transactions that require urgent review and approval.
When reviewing, approving, or ratifying a related person transaction, the Audit Committee will consider several factors,
including the benefits to the Company, the impact on a
director'sdirector’s independence in the event that a director or his/her immediate family is involved in the transaction, the terms of the transaction, and the terms available to unrelated third parties or to employees in general, if applicable. Related person transactions are approved only if the Audit Committee (or the Chairman of the Audit Committee pursuant to delegated authority in the circumstances noted above) determines that they are in, or are not inconsistent with, the best interests of the Company and our shareholders.Transactions with Related PersonsTRANSACTIONS WITH RELATED PERSONSCollaborations with Sanofi
As the beneficial owner of
23,353,66523,221,451 shares of common stock of the Company, or22.6%21.0% of the common stock outstanding as of April 14,2016,2020, Sanofi is considered a related person of the Company.In
2015,2019, Sanofi funded$145.0 million of our antibody discovery expenses under the Amended and Restated Discovery and Preclinical Development Agreement, and $747.8$964.3 million of our development and other costs (including$157.4$479.9 million of commercialization-relatedexpenses)expenses and $206.7 million in connection with reimbursements for manufacturing commercial supplies) under the Amended and Restated License and CollaborationAgreement.Agreement (the “Antibody License and Collaboration Agreement”). Inaddition, in 2015,2019, we also funded$92.6an aggregate of $46.0 million ofSanofi'sSanofi’s Phase 3 development costs forPraluent®Dupixent, Praluent, andsarilumabKevzara under theAmended and RestatedAntibody License and Collaboration Agreement. In2015,addition, in 2019, we and Sanofi sharedlossesprofits in connection with commercialization-related activities forPraluent® (which was launched in 2015 following receipt of regulatory approval in the United StatesDupixent, Praluent, andthe European Union) and sarilumab,Kevzara, which resulted in usfunding $240.0receiving $209.3 million of suchcosts.profits in the aggregate. In2016,2020, subject to the40 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING THE COMPANY / CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS following sentence, Sanofi has continued to fund the agreed-upon worldwide research and development expenses incurred by us and
Sanofi,Sanofi; we have continued to fund certain Phase 3 developmentcosts,costs; and we and Sanofi have continued to share certain commercialization-related revenues and expenses under theAgreements.In July 2015,Antibody License and Collaboration Agreement. Effective April 1, 2020, we and Sanofi amended the Antibody License and Collaboration Agreement to remove Praluent from the agreement such that, among other things, the agreement no longer governs the development, manufacture, or commercialization of Praluent; we and Sanofi entered intoa collaboration to discover, develop,the Praluent Cross License & Commercialization Agreement whereby we, at our sole cost, are solely responsible for the development andcommercialize antibody-based cancer treatmentscommercialization of Praluent in thefieldUnited States, and Sanofi, at its sole cost, is solely responsible for the development and commercialization ofimmuno-oncology (the "IO Collaboration"). The IO Collaboration is governed by an Immuno-oncology DiscoveryPraluent outside of the United States; andDevelopment Agreement (the "IO Discovery Agreement"), and an Immuno-oncologyunder the Praluent Cross Licenseand Collaboration Agreement (the "IO License and Collaboration Agreement"). In connection with the IO Discovery& Commercialization Agreement, Sanofimadepays us a$265.0 million non-refundable up-front payment to us in 2015. Pursuant to5% royalty on Sanofi’s net product sales of Praluent outside theIO Discovery Agreement, we will spend up to $1,090.0 million (the "IO Discovery Budget") to identify and validate potential immuno-oncology30United States until March 31, 2032.Certain Relationships and Related Transactionstargets and develop therapeutic antibodies against such targets through clinical proof-of-concept.In 2019, Sanofiwill reimburse us for up to $825.0 million of these costs, subject to certain annual limits. In connection with the IO License and Collaboration Agreement, Sanofi made a $375.0 million non-refundable up-front payment to us in 2015. Under the terms of the IO License and Collaboration Agreement, the parties willalsoco-develop our antibody product candidate targeting the receptor known as Programmed Cell Death protein 1, or PD-1 ("REGN2810"). The parties will share equally, on an ongoing basis, development expenses for REGN2810 up to a total of $650.0 million. In 2015, Sanofifunded$29.2$54.1 million of our research and development expenses under the Amended and Restated Immuno-oncology Discovery and Development Agreement (the “Amended IO DiscoveryAgreementAgreement”) and$10.8$108.9 million under theIOImmuno-oncology License and Collaboration Agreement.In
2013,addition, in 2019, Sanofi funded $10.3 million of commercialization-related expenses under the Immuno-oncology License and Collaboration Agreement and weacquiredrecognized $92.7 million of revenue that was previously deferred fromSanofi exclusive rights to antibodies targeting the platelet derived growth factor (PDGF) family of receptors and ligands.upfront payments received. In connection withthis arrangement, we made a $10.0 million development milestone payment to Sanofi in 2015.During 2014, Sanofi agreed to fund up to $17.5 million of agreed-upon costs incurred by us in connection with expanding manufacturing capacity at our Rensselaer, New York facility, ofthe entry into the Amended IO Discovery Agreement, which$13.2 had been received orwasreceivableeffective as of December 31,2015.2018, Sanofi also made a payment of $461.9 million to us in 2019 as consideration for (x) the termination of the parties’ original Immuno-oncology Discovery and Development Agreement (the “Original IO Discovery Agreement”), (y) the prepayment of certain discovery and development activities conducted by us regarding the development of certain therapeutic bi-specific antibodies, and (z) the reimbursement of costs we incurred under the Original IO Discovery Agreement during the fourth quarter of 2018. In 2020, we and Sanofi have continued to share certain development expenses for cemiplimab under the Immuno-oncology License and Collaboration Agreement (as amended effective as of January 7, 2018 by the letter agreement discussed below). In addition, if Sanofi elects to co-develop a therapeutic bi-specific antibody targeting BCMA and CD3 (a “BCMAxCD3 Program Antibody”) or MUC16 and CD3 (a “MUC16xCD3 Program Antibody”) under our immuno-oncology collaboration, Sanofi will initially fund almost all of the development expenses incurred in connection with the development of such BCMAxCD3 Program Antibody, for which Sanofi will be the principal controlling party, and half of the development expenses incurred in connection with the clinical development of such MUC16xCD3 Program Antibody, for which we will be the principal controlling party.A description of our antibody collaboration and our
IO Collaborationimmuno-oncology collaboration with Sanofi is set forth inPart II, Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" ofNote 3 to our2015audited financial statements for the fiscal year ended December 31, 2019 included in the 2019 Annual Report under the heading"Liquidity“a. Sanofi — Antibody” andCapital Resources – Collaborations with“a. Sanofi– Antibodies" and "–— Immuno-Oncology,"” respectively.In
February 2015,2019, we recorded $26.0 million of revenue primarily related to a percentage of net sales of ZALTRAP®(ziv-aflibercept) Injection for Intravenous Infusion and manufacturing ZALTRAP commercial supplies for Sanofientered into anunder the amended and restated collaboration agreement relating toZALTRAP® (ziv-aflibercept) Injection for Intravenous Infusion (the "Amended ZALTRAP® Collaboration Agreement"). Under the terms of the Amended ZALTRAP® Collaboration Agreement, Sanofi is solely responsible for the development and commercialization of ZALTRAP® for cancer indications worldwide. Sanofi bears the cost of all development and commercialization activities and reimburses Regeneron for its costs for any such activities. Sanofi will pay Regeneron a percentage of aggregate net sales of ZALTRAP® during each calendar year, which percentage shall be from 15% to 30%, depending on the aggregate net sales of ZALTRAP® in such calendar year. Regeneron is also paid for all quantities of ZALTRAP® manufactured by it pursuant to a supply agreement through the earlier of 2021 or the date Sanofi receives regulatory approval to manufacture ZALTRAP® at one of its facilities or a facility of a third party. Regeneron is no longer required to reimburse Sanofi for fifty percent of the development expenses that Sanofi previously funded for the development of ZALTRAP® under the original ZALTRAP® collaboration agreement. As aresult of entering into the Amended ZALTRAP® Collaboration Agreement, in 2015, we recognized $14.9 million of collaboration revenue, which was previously recorded as deferred revenue under the original ZALTRAP® collaboration agreement. In addition, during the year ended December 31, 2015, we recorded $38.8 million of revenue, primarily related to (i) revenues earned from Sanofi based on a percentage of net sales of ZALTRAP® and (ii) revenues earned from Sanofi for manufacturing ZALTRAP® commercial supplies.ZALTRAP.A description of the Amended ZALTRAP® Collaboration Agreement is set forth in Part I, Item 1. "Business" of our 2015 Annual Report, under the heading "Collaboration Agreements – Collaborations with Sanofi – ZALTRAP."Amended and Restated Investor Agreement with
SanofiSanofi; 2018 Letter AgreementIn January 2014, we entered into an Amended and Restated Investor Agreement with
Sanofi.Sanofi, which was subsequently amended effective as of January 7, 2018 by the letter agreement discussed below. Pursuant to theagreement,Amended and Restated Investor Agreement, Sanofi has agreed to vote its shares as recommended by our board of directors, except that it may elect to vote proportionally with the votes cast by all of our other shareholders with respect to certain change-of-control transactions and to vote in its sole discretion with respect to liquidation or dissolution of our company, stock issuances equal to or exceeding 20% of thethenoutstanding shares or voting rights of common stock and Class A stock (taken together), and new equity compensation plans or amendments if not materially consistent with our historical equity compensation practices.In addition,
upon Sanofi reaching 20% ownership of our then outstanding shares of Class A stock and common stock (taken together),wewereare required under theagreementAmended and Restated Investor Agreement to appoint an individual agreed upon by us and Sanofi to our board of directors. Subject to certain exceptions, we are required to use our reasonable efforts (including recommending that our shareholders vote in favor) to cause the election of this designee at our annual shareholder meetings for so long as (other than during the term of the letter agreement discussed below) Sanofi maintains an equity interest in us that is equal to thelower of (i) the highest percentage ownership Sanofi attains following its acquisition of 20% of our then outstanding shares of Class A stock and common stock (taken together) and (ii) 25% of our then outstanding shares of Class A stock and common stock (taken together)applicable Highest Percentage Threshold (as defined below). This designee is required to be"independent"“independent” of Regeneron, as determined underNASDAQNasdaq rules, and to nottobe a current or2019 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  /41
/41THE COMPANY / CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS former officer, director, employee, or paid consultant of Sanofi.
In April 2014,The current Sanofinotified us that it had reached the 20% ownership threshold and designated Robert A. Ingram as its designee. On April 4, 2014, following recommendation of the Corporate Governance and Compliance Committee,designee, Dr. Coles, was elected by the board of directorselected Mr. Ingram as a directorin January 2017 anda member ofby theCompensation Committee. Mr. Ingram was subsequently electedshareholders at the 2017 Annual Meeting as a ClassIII director,at our 2014 annual shareholder meetingand has been nominated by the board fora term expiringreelection at the2016 annual shareholder meeting and resigned on312020 Annual Meeting.Certain Relationships and Related TransactionsNovember 10, 2015. In December 2015, Sanofi disclosed in an amendment to its Schedule 13D filed with the SEC its intention to designate a successor designee.Under the Amended and Restated Investor Agreement, Sanofi also has three demand rights to require us to use all reasonable efforts to conduct a registered underwritten offering with respect to shares of our common stock held by Sanofi from time to time; however, shares of our common stock held by Sanofi from time to time may not be sold until
the later of (i)December 20, 2020or (ii)(other than with respect to an aggregate of 1,400,000 shares, as to which we have agreed to waive such “lock-up” during theexpirationterm ofour discoverythe letter agreement andpreclinical development agreement with Sanofi relatingof which 740,914 shares remained available toour antibody collaboration (as amended) if the agreement is extended beyond December 20, 2020.be sold as of April 14, 2020). These restrictions on dispositions are subject to earlier termination upon the occurrence of certain events, such as the consummation of a change-of-control transaction involving us or a dissolution or liquidation of Regeneron.Pursuant to the Amended and Restated Investor Agreement, Sanofi is bound by certain
"standstill"“standstill” provisions, which contractually prohibit Sanofi from seeking to directly or indirectlyexert control of Regeneron or acquiring more than 30% of our Class A stock and common stock (taken together). This prohibition will remain in place until the earliest of (i) the later of the fifth anniversaries of the expiration or earlier termination of our
licenseAntibody License andcollaboration agreementCollaboration Agreement with Sanofirelating to our antibody collaborationor ourZALTRAP®ZALTRAP collaboration agreement with Sanofi, each as amended; (ii) our announcement recommending acceptance by our shareholders of a tender offer or exchange offer that, if consummated, would constitute a change of control involving us; (iii) the public announcement of any definitive agreement providing for a change of control involving us; (iv) the date of any issuance of shares of common stock by us that would result in anotherparty'sparty’s having more than 10% of the voting power of ourthenoutstanding Class A stock and common stock (taken together) unless such party enters into a standstill agreement containing certain terms substantially similar to the standstill obligations of Sanofi; or (v) other specified events, such as a liquidation or dissolution of Regeneron.32Certain RelationshipsEffective January 7, 2018, we andRelated TransactionsTableSanofi and certain ofContents
Proposal No. 2: RatificationSanofi’s direct and indirect subsidiaries entered into a letter agreement in connection with (i) the increase ofAppointmentthe development budget amount for cemiplimab set forth in the Immuno-oncology License and Collaboration Agreement and (ii) the allocation ofIndependent Registered Public Accounting Firmadditional funds to certain proposed activities relating to the development of dupilumab and REGN3500 and non-approval trials of dupilumab (the “Dupilumab/REGN3500 Eligible Investments”). Pursuant to the letter agreement, we have agreed, among other things, to amend the definition of “Highest Percentage Threshold” to be the lower of (i) 25% of our outstanding shares of Class A stock and common stock (taken together) and (ii) the higher of (a) Sanofi’s percentage ownership of Class A stock and common stock (taken together) on the termination date of the letter agreement and (b) the highest percentage ownership Sanofi attains following such termination date of our outstanding shares of Class A stock and common stock (taken together); and to grant a limited waiver of Sanofi’s obligation to maintain the then-existing Highest Percentage Threshold during the term of the letter agreement in order to allow Sanofi to satisfy in whole or in part (a) its funding obligations with respect to the cemiplimab development costs under the Immuno-oncology License and Collaboration Agreement for the quarterly periods commencing on October 1, 2017 and ending on September 30, 2020 by selling up to 800,000 shares of our common stock directly or indirectly owned by Sanofi (of which 330,253 remained available as of April 14, 2020) and (b) its funding obligations with respect to the costs incurred by or on behalf of the parties to the Antibody License and Collaboration Agreement with respect to the Dupilumab/REGN3500 Eligible Investments for the quarterly periods commencing on January 1, 2018 and ending on September 30, 2020 by selling up to 600,000 shares of our common stock directly or indirectly owned by Sanofi (of which 410,661 remained available as of April 14, 2020). If Sanofi desires to sell shares of our common stock during the term of the letter agreement to satisfy a portion or all of its funding obligations for the cemiplimab development and/or Dupilumab/REGN3500 Eligible Investments, we may elect to purchase, in whole or in part, such shares from Sanofi. If we do not elect to purchase such shares, Sanofi may sell the applicable number of shares (subject to certain daily and quarterly limits) in one or more open-market transactions.42 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING
| THE COMPANY / CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
In 2019, Sanofi elected to sell, and we elected to purchase (either in cash or by issuing a credit towards the amount owed by Sanofi), an aggregate of 304,019 shares of our common stock pursuant to the letter agreement. In 2020 to date, Sanofi elected to sell, and we elected to purchase (either in cash or by issuing a credit towards the amount owed by Sanofi), an aggregate of 128,914 shares of our common stock pursuant to the letter agreement.
A more detailed description of the letter agreement with Sanofi is set forth in the 2019 Annual Report under Part I. Item 1. “Business —Collaboration Agreements — Collaborations with Sanofi.”
Stanford University
Effective September 1, 2016, Marc Tessier-Lavigne, Ph.D. became the President of Stanford University. In 2019, we made payments to Stanford University of approximately $162,000 in the aggregate relating to (i) services provided in connection with certain clinical trials entered into in the ordinary course of business and (ii) a medical education fellowship grant. In 2020 through the end of the first quarter, we made one payment to Stanford of less than $2,000 in respect of these services.
Indemnification of Directors and Officers
Our Certificate of Incorporation provides that, to the fullest extent permitted under the New York Business Corporation Law, no director or officer of our Company shall be personally liable to the Company or its shareholders for monetary damages for any breach of fiduciary duty in such capacity. In addition, our Amended and Restated By-Laws provide that we shall indemnify our directors and certain of our other personnel against expenses (including attorneys’ fees) and certain other liabilities, including judgments, fines, and amounts paid in settlement, arising out of or incurred as a result of legal actions brought or threatened against them by reason of their position in our Company, subject to certain qualifications and provided that each such person acted in good faith, in a manner that they reasonably believed was in our best interest, and, where applicable, not unlawful. Subject to the provisions of our Certificate of Incorporation, our Amended and Restated By-Laws, and the New York Business Corporation Law, we may also advance expenses of the individuals entitled to indemnification.
| 2019 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  /43 /43 |

| INTRODUCTION |
The Audit Committee has appointed PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for the fiscal year ending December 31, 2016.2020. PricewaterhouseCoopers LLP (or its predecessor) has audited the Company'sCompany’s financial statements for the past 2731 years.
The board of directors has directed that the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 20162020 be submitted for ratification by the shareholders at the Annual Meeting. Shareholder ratification of the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 20162020 is not required by the Company'sCompany’s charter documents or otherwise, but is being pursued as a matter of good corporate practice. If shareholders do not ratify the appointment of PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for fiscal year 2016,2020, the board of directors will consider the matter at its next meeting.
PricewaterhouseCoopers LLP has advised the Company that it will have in attendance at the 20162020 Annual Meeting a representative who will be afforded an opportunity to make a statement, if such representative desires to do so, and will respond to appropriate questions presented at the 20162020 Annual Meeting.
Information about Fees Paid to Independent RegisteredPublic Accounting Firm
| INFORMATION ABOUT FEES PAID TO INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
Aggregate fees incurred related to services provided to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 20152019 and 20142018 were:
| | 2015 | | 2014 | 2019 ($) | 2018 ($) | ||||
Audit Fees | $ | 1,721,000 | $ | 1,567,493 | 2,250,341 | 2,680,642 | |||
Audit-Related Fees | | 2,007 | | – | — | 85,000 | |||
| Tax Fees | 5,000 | 19,662 | |||||||
All Other Fees | | 4,637 | | 4,812 | 6,106 | 6,088 | |||
| | | | | | | | | ||
Total Fees | $ | 1,727,644 | $ | 1,572,305 | 2,261,447 | 2,791,392 | |||
Audit Fees.Audit fees in 20152019 and 20142018 were primarily for professional services rendered for the audit of the Company'sCompany’s financial statements for the fiscal year, including attestation services required under Section 404 of the Sarbanes-Oxley Act of 2002, technical accounting consultations related to the annual audit, and reviews of the Company'sCompany’s quarterly financial statements included in its Form 10-Q filings.
Audit-Related Fees.Audit-related fees in 20152018 were for professional services rendered forin connection with the review of a benefit plan of a foreign subsidiaryCompany’s adoption of the Company.new leasing accounting standard.
Tax Fees.Tax fees in 2019 and 2018 were for tax planning and advisory services.
All Other Fees.All other fees in 20152019 and 20142018 were for an annual subscriptionsubscriptions to a technical accounting database and for professional services rendered to a foreign subsidiary of the Company.resources.
44 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| THE COMPANY / AUDIT MATTERS |
The Audit Committee has adopted a policy regarding the pre-approval of audit and permitted non-audit services to be performed by the Company'sCompany’s independent registered public accounting firm, PricewaterhouseCoopers LLP. The Audit Committee will, on an annual basis, consider and, if appropriate, approve the provision of audit and non-audit services by PricewaterhouseCoopers LLP. The Audit Committee has approved a general provision of $75,000 for accounting advisory and other permissible consulting engagements. Management is responsible for notifying the Audit Committee of the status of accounting advisory and other permissible consulting engagements at regularly scheduled Audit Committee meetings and, if the Audit Committee so determines, the general provision is replenished to $75,000. The Audit Committee did not utilize thede minimisexception to the pre-approval requirements to approve any services provided by PricewaterhouseCoopers LLP during fiscal 20152019 and 2014.
The board of directors unanimously recommends a vote FOR ratification of the appointment of PricewaterhouseCoopers LLP as the Company's independent registered public accounting firm for the fiscal year ending December 31, 2016.
33
Proposal No. 2: Ratification of Appointment of Independent Registered Public Accounting Firm
![]()
| AUDIT COMMITTEE REPORT |
The Audit Committee Report below shall not be deemed to be "soliciting material" or to be filed with the SEC or subject to Regulation 14A or 14C under the Exchange Act, or to the liabilities of Section 18 of the Exchange Act. Notwithstanding anything to the contrary set forth in any of the Company's previous filings under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act that might incorporate future filings, including this proxy statement, in whole or in part, the Audit Committee Report below shall not be incorporated by reference into any such filings.
We have reviewed the audited financial statements of the Company for the year ended December 31, 2015,2019, which are included in the Company'sCompany’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015,2019, and met with both management and PricewaterhouseCoopers LLP, the Company'sCompany’s independent registered public accounting firm, to discuss those financial statements. The Audit Committee has discussed with the Company'sCompany’s independent registered public accounting firm the matters required to be discussed by Auditing Standard No. 16, as adopted bythe applicable requirements of the Public Company Accounting Oversight Board (the "PCAOB"“PCAOB”), which include, among other items, matters related to and the conduct of the audit of the Company's financial statements.Securities and Exchange Commission. The Audit Committee also discussed with the independent registered public accounting firm their independence relative to the Company and received and reviewed the written disclosures and the letter from the independent registered public accounting firm required by PCAOB Rule 3526 (Communication with Audit Committees Concerning Independence).the PCAOB.
Based on the foregoing discussions and review, the Audit Committee recommended to the board of directors that the audited financial statements of the Company for the year ended December 31, 20152019 be included in the Company'sCompany’s Annual Report on Form 10-K for the fiscal year ended December 31, 20152019 for filing with the Securities and Exchange Commission.
We have appointed PricewaterhouseCoopers LLP as the Company'sCompany’s independent registered public accounting firm for the fiscal year ending December 31, 2016.2020. This appointment was based on a variety of factors, including PricewaterhouseCoopers LLP'sLLP’s competence in the fields of accounting and auditing.
The Audit Committee
George L. Sing, Chairman
N. Anthony Coles, M.D.
Arthur F. Ryan
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  /45 /45 |

02
RATIFICATION OF
APPOINTMENT
OF INDEPENDENT
REGISTERED PUBLIC
ACCOUNTING FIRM
The board of directors unanimously recommends a voteFORratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2020.
46 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |

| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
The following table sets forth, as of April 14, 2020, the number of shares of the Company’s Class A stock and common stock beneficially owned by each of the Company’s directors, each of the NEOs referred to below under “Executive Compensation,” all directors and executive officers as a group, and each other person or group of persons known by the Company to beneficially own more than 5% of the outstanding shares of Class A stock or common stock, based upon (unless indicated otherwise) information obtained from such persons, and the percentage that such shares represent of the number of outstanding shares of Class A stock and common stock, respectively.
The Class A stock is convertible on a share-for-share basis into common stock. The Class A stock is entitled to ten votes per share and the common stock is entitled to one vote per share. We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, we believe, based on the information furnished or otherwise available to us, that the persons named in the table below have sole voting and investment power with respect to all shares of Class A stock and common stock shown as beneficially owned by them, subject to applicable community property laws. We have based our calculation of percentage of shares of a class beneficially owned on 1,848,970 shares of Class A stock and 110,673,311 shares of common stock outstanding as of April 14, 2020, except that for each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock beneficially owned by that person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person assume the conversion on April 14, 2020 of all shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group) into common stock and also that no other shares of Class A stock beneficially owned by others are so converted.
In computing the number of shares of common stock beneficially owned by a person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person (and by directors and executive officers as a group), shares of common stock subject to options, PSUs, RSUs, or other convertible securities (if any) held by that person (and by directors and executive officers as a group) that are exercisable or releasable as of April 14, 2020 or are exercisable or releasable within sixty days after April 14, 2020 are deemed to be outstanding. Such shares are not deemed to be outstanding, however, for the purpose of computing the percentage ownership of common stock of any other person.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 47 / 47 |
| SHAREHOLDERS |
| Shares of Class A Stock Beneficially Owned1 | Shares of Common Stock Beneficially Owned1 | |||||||||||
| Name and Address of Beneficial Owner | Number | Percent of Class | Number2 | Percent of Class | ||||||||
| Beneficial Owners of More than 5% of Class A Stock or Common Stock (Other than Directors and Executive Officers): | ||||||||||||
| Sanofi3 | ||||||||||||
| 54, rue La Boetie 75008 Paris, France | — | — | 23,221,451 | 21.0 | % | |||||||
| Capital World Investors4 | ||||||||||||
| 333 South Hope Street Los Angeles, California 90071 | — | — | 8,351,951 | 7.5 | % | |||||||
| The Vanguard Group, Inc.5 | ||||||||||||
| 100 Vanguard Blvd. Malvern, PA 19355 | — | — | 6,252,303 | 5.6 | % | |||||||
| BlackRock, Inc.6 | ||||||||||||
| 55 East 52nd Street New York, New York 10055 | — | — | 6,138,848 | 5.5 | % | |||||||
| Directors and Executive Officers:7 | ||||||||||||
| Leonard S. Schleifer, M.D., Ph.D. | 1,726,5658 | 93.4% | 4,127,724 | 9 | 3.6 | % | ||||||
| P. Roy Vagelos, M.D. | — | — | 1,821,406 | 10 | 1.6 | % | ||||||
| George D. Yancopoulos, M.D., Ph.D. | 42,75011 | 2.3% | 2,520,436 | 12 | 2.2 | % | ||||||
| N. Anthony Coles, M.D. | — | — | 23,835 | 13 | 26 | |||||||
| Bonnie L. Bassler, Ph.D. | — | — | 28,930 | 14 | 26 | |||||||
| Michael S. Brown, M.D. | — | — | 56,618 | 15 | 26 | |||||||
| Joseph L. Goldstein, M.D. | — | — | 48,019 | 16 | 26 | |||||||
| Andrew J. Murphy, Ph.D. | — | — | 330,913 | 17 | 26 | |||||||
| Christine A. Poon | — | — | 122,089 | 18 | 26 | |||||||
| Arthur F. Ryan | — | — | 80,819 | 19 | 26 | |||||||
| George L. Sing | — | — | 224,141 | 20 | 26 | |||||||
| Marc Tessier-Lavigne, Ph.D. | — | — | 67,956 | 21 | 26 | |||||||
| Robert E. Landry | — | — | 158,891 | 22 | 26 | |||||||
| Daniel P. Van Plew | — | — | 382,430 | 23 | 26 | |||||||
| Huda Y. Zoghbi, M.D. | — | — | 28,930 | 24 | 26 | |||||||
| All Directors and Executive Officers as a Group (19 persons) | 1,769,315 | 95.7% | 10,917,813 | 25 | 9.2 | % | ||||||
| 1 | The inclusion in this table of any Class A stock or common stock, as the case may be, deemed beneficially owned does not constitute an admission of beneficial ownership of those shares. | ||
| 2 | For each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock listed includes the number of shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group). | ||
| 3 | Based solely on a Form 4 filed by Sanofi with the SEC on March 11, 2020. According to this Form 4, 20,421,899 of the shares are held directly by Sanofi and 2,799,552 of the shares are held directly by Aventisub LLC (formerly known as Aventis Pharmaceuticals Inc.). Aventisub LLC is an indirect, wholly-owned subsidiary of Sanofi. Pursuant to the Amended and Restated Investor Agreement, dated as of January 11, 2014 (as amended), by and among Sanofi, sanofi-aventis US LLC, Aventisub LLC, and sanofi-aventis Amérique du Nord (collectively, the “Sanofi Parties”), and the Company, the Sanofi Parties have agreed to vote all shares of our voting securities they are entitled to vote from time to time as recommended by our board of directors, except that they may elect to vote proportionally with the votes cast by all of our other shareholders with respect to certain change-of-control transactions and to vote in their sole discretion with respect to liquidation or dissolution of Regeneron, stock issuances equal to or exceeding 20% of the outstanding shares or voting rights of Class A stock and common stock (taken together), and new equity compensation plans or amendments if not materially consistent with our historical equity compensation practices. See “The Company — Certain Relationships and Related Transactions — Transactions with Related Persons — Amended and Restated Investor Agreement with Sanofi; 2018 Letter Agreement” above for further information regarding the Amended and Restated Investor Agreement with Sanofi. |
48 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| SHAREHOLDERS |
| 4 | Based solely on an amendment to a Schedule 13G filed by Capital World Investors on February 12, 2020. According to this amendment, Capital World Investors, a division of Capital Research and Management Company (“CRMC”), has sole voting as to 8,308,528 of the shares reported as beneficially owned and dispositive power as to all of the shares reported as beneficially owned. | ||
| 5 | Based solely on an amendment to a Schedule 13G filed by The Vanguard Group, Inc. on February 10, 2020. According to this amendment, The Vanguard Group, Inc. has sole voting power as to 120,348, shared voting power as to 23,068, sole dispositive power as to 6,116,133, and shared dispositive power as to 136,170 of the shares reported as beneficially owned. Vanguard Fiduciary Trust Company, a wholly-owned subsidiary of The Vanguard Group, Inc., is the beneficial owner of 91,833 shares as a result of its serving as investment manager of collective trust accounts. Vanguard Investments Australia, Ltd., a wholly-owned subsidiary of The Vanguard Group, Inc., is the beneficial owner of 71,498 shares as a result of its serving as investment manager of Australian investment offerings. | ||
| 6 | Based solely on an amendment to a Schedule 13G filed by BlackRock, Inc. on February 5, 2020. According to this amendment, BlackRock, Inc. has sole voting power as to 5,367,704 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. | ||
| 7 | The address for each director and executive officer is c/o Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707. | ||
| 8 | Includes 15,775 shares of Class A stock held in trust for the benefit of Dr. Schleifer’s son, of which Dr. Schleifer is a trustee. | ||
| 9 | Includes (i) 1,820,844 shares of common stock purchasable upon the exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan, the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, or the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (collectively, the “Long-Term Incentive Plans”) that are exercisable or become so within sixty days after April 14, 2020; (ii) 100,000 shares of common stock held in a grantor retained annuity trust of which Dr. Schleifer is the trustee; and (iii) 5,816 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 10 | Includes (i) 1,145,495 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 2,204 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iii) 143,900 shares of common stock held in a charitable lead annuity trust, of which Dr. Vagelos is the trustee; (iv) 83,652 shares of common stock held in a trust for his grandchildren, of which Dr. Vagelos’s wife is the trustee; (v) 1,203 shares of common stock held in trusts for his grandchildren, of which Dr. Vagelos and/or his wife are trustees; and (vi) 54,146 shares of common stock and 86,321 shares of common stock held by the Marianthi Foundation and the Pindaros Foundation, respectively, both of which are charitable foundations of which Dr. Vagelos is a director and an officer. Dr. Vagelos disclaims beneficial ownership of the shares held by these charitable foundations. | ||
| 11 | Of these shares, 23,367 shares are held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members; Dr. Yancopoulos is a trustee of the trust. The remaining 19,383 shares are held in custody for the benefit of Dr. Yancopoulos’s children. | ||
| 12 | Includes (i) 1,371,988 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 5,791 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iii) 557,465 shares held in separate grantor retained annuity trusts, of which Dr. Yancopoulos is the trustee; (iv) 517,799 shares of common stock held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members, of which Dr. Yancopoulos is a trustee; and (v) 24,643 shares of common stock held in trusts for the benefit of Dr. Yancopoulos’s children. | ||
| 13 | Consists of (i) 23,370 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 14 | Consists of (i) 28,465 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 15 | Consists of (i) 38,804 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020, which are held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (ii) 11,349 shares of common stock held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (iii) 5,000 shares of common stock held in a trust of which Dr. Brown’s spouse is trustee for the benefit of Dr. Brown’s immediate family members; (iv) 1,000 shares of common stock held by a family charitable foundation of which Dr. Brown is a director and an officer and his wife is a director; and (v) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. Dr. Brown disclaims beneficial ownership of the shares referenced in (iii) and (iv). | ||
| 16 | Includes (i) 38,554 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 17 | Includes (i) 276,750 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 27,625 shares of restricted stock (“RSAs”); and (iii) 4,212 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 18 | Includes (i) 120,834 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 19 | Includes (i) 52,054 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 20 | Includes (i) 93,804 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 2,400 shares of common stock held by Mr. Sing’s spouse; (iii) 3,700 shares of common stock held by Mr. Sing’s spouse as custodian for the benefit of their son; (iv) 9,000 shares of common stock held in a trust for benefit of Mr. Sing’s son; and (v) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 21 | Includes (i) 66,304 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  /49 /49 |
| SHAREHOLDERS |
| days after April 14, 2020 upon termination of service. | |||
| 22 | Includes (i) 133,493 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 20,125 RSAs; and (iii) 172 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 23 | Includes (i) 352,642 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 7,625 RSAs; and (iii) 1,537 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 24 | Consists of (i) 28,465 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; and (ii) 465 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020 upon termination of service. | ||
| 25 | Includes (i) 6,397,730 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 14, 2020; (ii) 83,108 RSAs; (iii) 4,185 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 14, 2020; and (iv) 27,183 shares of common stock held in an account under the Company’s 401(k) Savings Plan. | ||
| 26 | Represents less than 1%. |
| SHAREHOLDER COMMUNICATIONS |
The Company has established a process for shareholders to send communications to the members of the board of directors. Shareholders may send such communications by mail addressed to the full board, a specific member or members of the board, or a particular committee of the board, at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, Attention: Corporate Secretary. All such communications will be opened by our Corporate Secretary for the sole purpose of determining whether the contents represent a message to our directors. Any contents that are not in the nature of advertising, promotions of a product or service, or patently offensive material will be forwarded promptly to the addressee. In the case of communications to the board or any individual director or group or committee of directors, the Corporate Secretary will make sufficient copies of the contents to send to such director or each director who is a member of the group or committee to which the envelope is addressed.
50 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |

| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 51 / 51 |
34
Audit Committee Report
Executive Compensation
2020 COMPENSATION-RELATED PROPOSALS
This year, we ask you to cast your vote on two compensation-related proposals:
| I | We seek your authorizationfor a sufficient number of shares to fund our compensation program (Proposal No. 3).1 |
| II | We seek your inputon our approach to compensation for our “Named Executive Officers”2through the advisory vote commonly referred to as “say on pay” (Proposal No. 4). |
To best understand these proposals, it is important to consider our overall approach to compensation and certain key factors that play a significant role in shaping the program’s structure and design. These factors include how closely we tie our pay practices to our mission, strategy, and business model; our ongoing, company-wide pay philosophy; and our process for seeking and carefully considering valuable shareholder feedback each year.
As you decide how to vote on these proposals, please keep in mind the following:
Key Takeaways
| 1 | Our equity compensation program supportsall of our employees, not just our NEOs, with approximately 90% of recent annual equity grants awarded to employees other than our NEOs. |
| 2 | We granted performance-based restricted stock units (“PSUs”) to our CEO and CSO as a component of their 2019 annual equity awards. This and other carefully calibrated changes to our compensation program were based on investor feedback. |
| 3 | Our 2019 burn rate was at the lowest level in the last seven years due to specific steps we took to manage dilution from equity compensation. |
| 4 | We are asking our shareholders to approve the same number of shares for equity grants that we requested previously and are committed to ensuring that shares available under our long-term incentive plan will be sufficient for at least three years. |
| 5 | Our compensation model underpins our strategy of creating and advancing a high-quality, internally developed product pipeline, which delivered six important medicines and eight additional key indications for these products in the past decade. |

| 1 | In this section, “we,” “us,” and “our” refer to the Company and, where applicable, to the Compensation Committee of the Company’s board of directors. |
| 2 | Our “Named Executive Officers” or “NEOs” are identified below under “Compensation Discussion and Analysis.” |
52 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATEDMATTERS / INTRODUCTION |
Our Corporate Culture
Stability, consistency, and an unrelenting focus on science and innovation have been important to our Company’s success and long-term approach. Regeneron’s culture is defined by loyal and motivated employees with an entrepreneurial spirit who are dedicated to the Company’s mission to use the power of science to invent medicines for people with serious diseases. This dedication allowed us to bring six important, internally developed medicines to patients and obtain eight additional key indications for these products in the past decade. We have also consistently outperformed the industry in employee retention in recent years, as demonstrated by our employee attrition rate of less than half the industry average in each of the last five years.3
We continue to be led by Leonard S. Schleifer, M.D., Ph.D., one of the longest-serving founder CEOs in the S&P 500, and George D. Yancopoulos, M.D., Ph.D., our co-founder and Chief Scientific Officer. Drs. Schleifer and Yancopoulos, along with our management and scientific teams, have been the stewards of our long-term approach and are part of the formula for our success. The Company’s dedication to science is also reflected in a board of directors and senior management team that are both heavily populated with top scientific talent.
We firmly believe that our compensation model that emphasizes long-term performance has helped us establish a culture where employees focus on our mission and drug discovery and development. Managing our business for the long term is a core Company belief, as demonstrated by our history of growing through innovation and through a pipeline of internally developed medicines. It also reflects our philosophy of “doing well by doing good,” which encompasses our approach to operating responsibly; making drug-pricing decisions with fairness, affordability, and access at the forefront; and our commitment to patients.
Our Pay Philosophy
Our compensation program supports our core strategy of creating and advancing a high-quality, internally developed product pipeline. The pipeline is directly created by our talented employees, and their engagement, commitment, and achievements are key drivers of pipeline success and therefore our long-term performance.
The primary underpinning of our pay philosophy is to award equity-based pay toall eligible employees to ensure that when we deliver for patients and for shareholders, everyone shares in the upside growth. We award initial equity grants to all new hires, primarily in the form of stock options, in addition to a comprehensive annual equity program. In each of the last five years, approximately 90% of the employee equity grants were awarded to our employees other than our NEOs.4We believe that our broad-based equity program, which emphasizes long-term performance and ensures that all employees can share in our future potential, represents one of our competitive business advantages.
In each of the last five years, approximately90%of employee equity grants were awarded to employees other than our NEOs.
We have primarily used stock options for our employee equity awards because we think stock options are easy to understand, are long-term focused (consistent with the drug discovery and development cycle), and are difficult to manipulate by “managing to metrics.” Importantly, stock options are completely aligned with shareholders’ interests. We also believe that the performance-based nature of stock options is enhanced for companies like Regeneron whose stock price is directly impacted by pipeline developments.
We periodically assess our approach to compensation in light of our business objectives, feedback from our shareholders, and market practices in order to adjust our compensation program based on evolving business needs. As discussed further below, in 2019 we introduced PSUs as a component of the annual equity awards for Regeneron’s
| 3 | For example, our 2019 turnover rate was 7.8% compared to an industry average of 18.7%, with turnover in our research & development organization ranking lowest of all employee groups. Industry average is based on the Radford U.S. Life Sciences Trends Report for 2019. |
| 4 | Based on both the grant date fair value and the number of underlying shares (on an options-equivalent basis). Unless otherwise noted, award percentages below are based on the number of underlying shares (on an options-equivalent basis). |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 53 / 53 |
| COMPENSATION-RELATEDMATTERS / INTRODUCTION |
CEO and CSO (as well as the Chairman of the Board). In addition, we introduced restricted stock (“RSAs”) or restricted stock units (“RSUs”) for all other eligible employees as a component of their annual equity awards. While stock options continue to represent a key component of our equity program, we also believe that utilizing a mix of stock options and RSAs/RSUs will provide flexibility for managing dilution and promoting employee retention.
Shareholder Engagement and Feedback
We believe our compensation model is appropriate for our Company and is supported by our long-term performance, but also think it is very important to reach out to our shareholders for ideas, input, and direct feedback. We do this formally through our triennial say-on-pay vote, as well as through direct discussions with our shareholders and informal exchanges in other settings.
Our annual shareholder outreach program is quite extensive – last year we approached shareholders collectively representing nearly 60% of the shares of common stock outstanding as of December 31, 2019 (excluding shares held by our directors and executive officers and Sanofi), which we refer to as “public shareholders”. As part of our outreach, we engaged in direct one-on-one discussions with shareholders representing over 50% of our public shareholders as well as both leading proxy advisory firms. Our Compensation Committee Chair led many of these discussions. The feedback from shareholders has resulted in specific changes to our compensation and corporate governance practices and policies, including the changes discussed below.
Changes that took into account feedback from recent shareholder engagement include the following:
| What We Heard | What We Have Done | |
| Preference for further pay-for-performance alignment | Granted PSUs to CEO and CSO in 2019 with rigorous performance goals tied to theCompany’s absolute total shareholder return (“TSR”) (target = 61% TSR over five years). | |
| Desire for more clarity regardingthe process for determining annualcash incentives | Enhanced the existing process for determining 2019 annual cash incentives and providedmore detailed disclosure about the Compensation Committee’s determination of theCompany performance multiplier in the CD&A section of this proxy statement.* | |
| Support for all-employee equitycompensation strategy, butconcern about resulting burn rate | Maintained our all-employee equity compensation strategy while addressing concernsabout the resulting burn rate using a two-part approach:
• Introduce full-value awards for a portion of the annual equity awards to eligible employees. | |
| Concern about compensationprogram for non-employeedirectors and the Chairman of theBoard | Introduced a new compensation program for our non-employee directors and the Chairmanof the Board in November 2018. As a result: • Reduced by nearly 50% the grant date fair value of equity awards to both the non-employee directors and the Chairman of the Board (granted in January 2019 and December 2018, respectively); and • Introduced full-value awards (RSUs) as part of the non-employee director equity awards. |
| * | See “Compensation Discussion and Analysis – Components of Executive Pay: What We Pay and Why We Pay It – Annual Cash Incentives” for more information. |
These are just the latest examples of how our compensation program is continually refined. See the subsection “Compensation Discussion and Analysis – Our Compensation Processes – Shareholder Input and Outreach” for additional information on changes recently adopted based on shareholder feedback.
54 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATEDMATTERS / INTRODUCTION |
Our Compensation Program Today: Aligning Pay and Performance
We believe that our compensation program today is effective and aligns with our culture, pay philosophy, and shareholder input and interests.
Significant Performance-Based (“At-risk”) Pay:At-risk pay accounts for a high proportion of compensation for our NEOs and other senior executives, which has been a consistent and defining feature of our program. For our CEO, over 93% of his direct pay5is at-risk and directly correlated to performance; in contrast, the average percentage of CEO at-risk compensation in our Peer Group is significantly lower, at 86%.6
Use of PSUs for CEO and CSO:As discussed above, in 2019 we introduced long-term PSUs to account for 30% of the annual equity awards of our CEO and CSO, which we believe will further incentivize our top executives to drive long-term, sustainable, and exceptional shareholder value creation.
Continual Management of Dilution:Over the last seven years, we have managed dilution from equity compensation by (i) reducing by nearly 60% the number of shares underlying the annual equity awards of our eligible employees, including our CEO; and (ii) adding time-based RSAs/RSUs to the annual long-term incentive mix for our employees (other than those receiving PSUs). Thanks to these measures, 2019 burn rate was at the lowest level in the last seven years. We did this while still maintaining our all-employee equity award strategy and concurrently creating over 6,000 new full-time jobs.7
In the last decade, we sought shareholder approval of additional shares for our long-term incentive plans three times. Each time, we requested the same number of shares – 12 million – and lived up to our commitment not to seek additional shares earlier than three years following approval. Once again, we are asking shareholders to approve 12 million additional shares for equity grants and are committed to ensuring that shares available under our long-term incentive plan will be sufficient for at least three years.
Regeneron Stock Utilization vs. Headcount*

ThisRegeneron’s 2019 burn rate was at the lowest level in the last seven years.
| * | Burn rate calculated by dividing the number of shares subject to equity awards (stock options, RSAs, and RSUs) granted during the year by the basic weighted-average number of shares of common stock and Class A stock outstanding during the year. PSUs are to be reflected in the burn rate calculation for the year in which they are earned and, therefore, do not impact the burn rate shown in the chart. Headcount numbers based on the number of employees as of December 31 of the applicable year. |
Alignment of Pay with Performance:As stock options comprise most of our CEO’s reported compensation and do not provide value unless there is positive shareholder return, such compensation is closely aligned with the Company’s performance. The charts below illustrate this alignment based on both (i) the as-reported grant date fair value and (ii) a realizable pay metric.
| 5 | We define “direct pay” or “direct compensation” as total compensation as reported in the Summary Compensation Table in the applicable proxy statement, other than the amounts reported as “All other compensation” and (if applicable) amounts reported under “Change in pension value and nonqualified deferred compensation earnings.” |
| 6 | Based on the Peer Group median for each compensation component (using data reported for 2018). See the subsection “Compensation Discussion and Analysis – Our Compensation Processes — Peer Data” below for a list of the companies included in our Peer Group. |
| 7 | In 2019, we added 730 new jobs and yet reduced the burn rate for that year by 1.1%, from 4.7% to 3.6%. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 55 / 55 |
| COMPENSATION-RELATEDMATTERS / INTRODUCTION |
| 1 | Alignment Based on Grant Date Fair Value.Over the last five years, our CEO’s total direct compensation tracked our TSR, as shown below. |
CEO Total Direct Compensation vs. Indexed TSR*
($ millions)
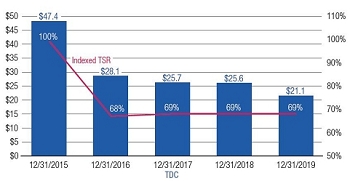
| * | “Total direct compensation” or “TDC” means total compensation as reported in the Summary Compensation Table in the applicable proxy statement (other than amounts reported as “All other compensation”). “Indexed TSR” in the period presented in this chart is expressed as a percentage of the closing price of our common stock on 12/31/2015. |
| 2 | Alignment Based on Realizable Pay.Our CEO’s realized/realizable pay from compensation awarded over the last five years was significantly lower than the values reported for that compensation ($20.3 million of realized/realizable pay versus $148.0 million of reported value). Nearly all of the realized/realizable value was from salary and bonus because there was almost no value provided from equity awards granted during this five-year period. The chart below compares the aggregate, as-reported value of our CEO’s total direct compensation over the last five years with his realized/realizable pay during the same period. We believe this demonstrates that our CEO compensation design rewards performance and, conversely, limits payouts in respect of periods with flat or negative TSR (as was the case in the period depicted below). |
Five-Year CEO Total Direct Compensation – Reported vs. Realized/Realizable*
($ millions)
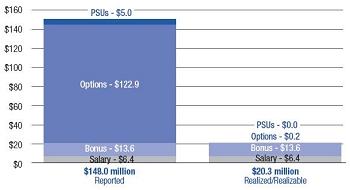
| * | “Realized/realizable pay” means, for any measurement period, the sum of the (i) as-reported base salaries and annual cash incentives paid during such period, (ii) amounts realized as a result of the exercise of stock options awarded during such period, and (iii) amounts that could have been realized as a result of the exercise of outstanding and “in-the-money” stock options or the sale of shares delivered upon vesting of other equity awards, in each case awarded during such period (using the closing price of our common stock on the last trading day of the period). |
56 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |

COMPENSATION DISCUSSION AND ANALYSIS
The following Compensation Discussion and Analysis provides(“CD&A”) describes the philosophy, objectives, and structure of our fiscal year 2019 executive compensation program. This CD&A is intended to be read in conjunction with the subsequent tables presented under “Compensation Dashboard – 2019 Executive Compensation Tables,” which provide further historical compensation information aboutfor our 2015“Named Executive Officers” or “NEOs”.8

Our compensation program underpins our strategy of delivering sustainable, long-term growth through continued innovation. We believe the success of our compensation program and our performance (both financial and operational, in particular with respect to our pipeline progress) are best judged from a long-term perspective. The past decade was a transformational period for Regeneron – the following executive officers (the "Named Officers"), whose compensation is set forthCompany evolved from a small biotech with a mostly pre-commercial portfolio to a large-cap company with seven commercialized products, including two with global annual net product sales of over $2 billion; a pipeline with multiple promising product candidates (including a comprehensive immuno-oncology program); a deep bench of scientific and industry talent; proprietary platform technologies that help accelerate and improve the traditional drug development process; one of the largest genetics sequencing efforts in the Summary Compensation Table
world; and a strong balance sheet with capital to advance and expand our wholly-owned R&D pipeline. We are proud that in that period we brought six important, internally developed medicines to patients and obtained eight additional key indications for these products. In the other compensation tables included in this proxy statement:most recent period, we made further progress and celebrated significant scientific, operational, and financial accomplishments, as discussed further under “2019 Business Highlights” below.
8 | ||
| These are determined in accordance with SEC rules, and for this year consist of our President and Chief Executive Officer | ||
| (“CEO”); President | ||
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 57 / 57 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
To keep key managers motivated and focused, we believe that we must continue to provide an executive compensation package that prioritizes achievement of our long-term goals (particularly those related to drug discovery and development), incentivizes high performance, and strongly aligns with the direct interests of our long-term investors.
Compensation decisions of the past year balance consistency and flexibility following a thoughtful process for setting CEO and other NEO compensation. We believe the resulting pay outcomes were commensurate with 2019 performance and the NEOs’ contributions in 2019 and will incentivize performance in the years to come.
2019 COMPENSATION HIGHLIGHTS
Select compensation highlights from the past year include the following:
Base Salaries
Annual Cash Incentives
| • | (New)In response to shareholder input, we enhanced the existing process by which the Compensation Committee determines the annual cash incentive Company performance multiplier and provided additional disclosure about the Committee’s decision-making (see “Components of Executive Pay: What We Pay and Why We Pay It – Annual Cash Incentives” below for additional information). |
| • | Following a year with a great deal of clinical and operational success, the annual cash incentives paid out at 177% of each NEO’s target bonus, on average, in 2019. |
Annual Equity Awards
| • | (New)In December 2019, following feedback from shareholders and in light of other relevant considerations, we granted PSUs as part of the 2019 annual equity award to our CEO and CSO (as well as the Chairman of the Board). The PSUs are long-term awards intended to motivate exceptional shareholder value creation over the next five years. The PSUs accounted for approximately 30% of each officer’s 2019 annual equity awards, while the remaining 70% consisted of stock options. Key features of the PSUs, which are discussed more fully below under “Components of Executive Pay: What We Pay and Why We Pay It — Annual Equity Awards” below, include: |
| • | Long-term, five-year performance period (with an opportunity for accelerated payout after four years if performance is strong); and | |
| • | Rigorous performance goals tied to the Company’s TSR over the performance period (target is a 61% TSR over five years and minimum threshold payout requires at least a 28% TSR). |
2019 BUSINESS HIGHLIGHTS
Our corporate performance for 2019 was assessed in three categories: (1) factors related to our product pipeline and development for both the near- and long-term; (2) factors regarding our financial performance and operations; and (3) factors related to our talent, culture, and corporate responsibility.
As in prior years, the factors analyzed under category (1) continued to be a key measure for the Compensation Committee’s assessment and calculating the Company performance multiplier. This recognizes the importance of science and innovation as well as the critical role of the development pipeline in the Company’s long-term success.
We provide a comprehensive overview of our 2019 achievements and the Compensation Committee’s assessment of those achievements in the subsection “Components of Executive Pay: What We Pay and Why We Pay It — Annual Cash Incentives.”
58 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
Select 2019 achievements are highlighted in the table below.
| Regulatory Approvals | ||
• EYLEA:U.S. Food and Drug Administration approvals for a new indication, diabetic retinopathy, and pre-filled syringe • Dupixent: • FDA and European Commission approvals for expanded atopic dermatitis indication in adolescent patients (12–17 years of age) • FDA and European Commission approvals for a new indication, chronic rhinosinusitis with nasal polyposis • European Commission approval for the treatment of asthma in adults and adolescents • Libtayo:Conditional European Commission approval for the treatment of advanced cutaneous squamous cell carcinoma | • Dupixent:Completed Phase 3 study in pediatric patients (6–11 years of age) with severe atopic dermatitis • Libtayo:Interim objective response rate readout from Phase 3 study in non-small cell lung cancer • REGN1979(bispecific antibody targeting CD20 and CD3): Reported data from non-Hodgkin lymphoma study • REGN5458(bispecific antibody targeting BCMA and CD3): Reported initial data from multiple myeloma study • REGN-EB3(multi-antibody therapy to Ebola virus infection): Shown to be superior to ZMapp in preventing death | |
| Commercial Execution |
It is organized into the following sections:
•Section 1 – Summary (page 35)•Section 2 – Analysis of 2015 Executive Compensation Based on Compensation Objectives (page 40)
•Section 3 – Executive Compensation Process and Considerations (page 49)•Section 4 – Elements of Executive Compensation (page 54)
• Full-year 2019 global net product sales of $7.54 billion* • Fourth quarter 2019 U.S. net product sales increased 13% to $1.22 billion versus fourth quarter 2018 • Dupixent:Full-year 2019 global net product sales of $2.32 billion** • Libtayo:#1 systemic treatment in cutaneous squamous cell carcinoma in the United States • Antibody Collaboration with Sanofi: •Achieved profitability for the first time on a quarterly basis in the second quarter of 2019 • Increased profitability in the third and fourth quarters of 2019 | • Revenue:Full-year 2019 revenues increased 17% to $7.86 billion versus 2018*** • Non-GAAP Diluted EPS:Non-GAAP net income per share, or EPS, for full-year 2019 increased 8% versus 2018**** • Business Development:Approximately $850 million of equity investments and upfront payments in connection with our collaboration arrangements • Share Repurchase Program:Initiated a share repurchase program to repurchase up to $1.0 billion of our common stock |
2015 Performance Overview
We are a fully integrated biopharmaceutical company that discovers, invents, develops, manufactures, and commercializes medicines for*Our collaborator Bayer records net product sales of EYLEA outside the treatment of serious medical conditions. We commercialize medicines for eye diseases, high LDL cholesterol, and a rare inflammatory condition and have product candidates in development in other areas of high unmet medical need, including oncology, rheumatoid arthritis, asthma, atopic dermatitis, pain, and infectious diseases. For more information about our business, please see Part I, Item 1. "Business" and Part II, Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" of our 2015 Annual Report.United States.
2015 was another extraordinary year for Regeneron.
**Our key accomplishments in 2015 included:
•47% growth in EYLEA® (aflibercept) Injectioncollaborator Sanofi records global net product salesas comparedof Dupixent.***As reported in the 2019 Annual Report. In the second quarter of 2020, Regeneron announced that it had implemented changes in the presentation of its consolidated financial statements relating to
2014,certain reimbursements and other payments for products developed and commercialized with collaborators. These changes were made effective January 1, 2020 and have also been applied retrospectively. After giving effect to these changes, Regeneron’s revenue for fiscal 2018 and fiscal 2019 would have been $5.15 billion and $6.56 billion, respectively. There is no impact from$2.775 billionthese changes to$4.089 billion.•46% growth in our total revenuesas compared to 2014, from $2.820 billion to $4.104 billion.
•19% growth innet income or net income per share.****Non-GAAP net income and non-GAAP net income
as compared to 2014, from $1.175 billion to $1.404 billion. (Non-GAAP net income isper share, or EPS, are nota measuremeasures calculated in accordance with U.S. Generally Accepted AccountingPrinciples.Principles (“GAAP”). See Appendix A for a definition ofnon-GAAP net incomethese measures and a reconciliation ofnon-GAAP net income to net income.)•Advances in our EYLEA® franchise, including:oRegulatory approvaleach ofEYLEA® for the treatment of visual impairment due to macular edema secondary to retinal vein occlusion and the treatment of visual impairment secondary to myopic choroidal neovascularization in the European Union;oRegulatory approval of EYLEA® for the treatment of diabetic retinopathy in patients with diabetic macular edema in the United States; andoRegulatory approval of EYLEA® for the treatment of retinal vein occlusion in Japan.
•Regulatory approval and launch of Praluent® (alirocumab) Injection, the first FDA-approved drug in a new class of drugs that lower LDL ("bad") cholesterol.
35
Executive Compensation
•Completion of enrollment in the Praluent® ODYSSEY OUTCOMES trial, an 18,000-patient study prospectively assessing the potential of Praluent® to demonstrate cardiovascular benefit.•Further advances in our late-stage clinical pipeline, which includes sarilumab, an IL-6 receptor antibody for rheumatoid arthritis; dupilumab, an IL-4 receptor-alpha antibody for allergic diseases; fasinumab, an antibody to nerve growth factor (NGF); and REGN2222, an antibodythese measures to theRespiratory Syncytial Virus-F (RSV-F) protein:most directly comparable GAAP financial measure.2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING  / 59
/ 59
COMPENSATION-RELATED MATTERS /
COMPENSATION DISCUSSION AND ANALYSIS
oReported positive Phase 3 data for sarilumab from three Phase 3 studiesTSR Performance
Despite progress in
patients with rheumatoid arthritis (SARIL-RA-TARGET, SARIL-RA-EASY, and SARIL-RA-ASCERTAIN) and submitted a Biologics License Application for sarilumab with the FDA.oReported positive pivotal Phase 2b data for dupilumab in asthma and completed enrollment of the dupilumab atopic dermatitis Phase 3 studies.oEntered into a collaboration agreement relating to fasinumab with Mitsubishi Tanabe Pharma Corporation for Japan, Korea, and nine other Asian countries, excluding China.oInitiated a Phase 3 clinical study of REGN2222 for Respiratory Syncytial Virus.
•Further progress of Regeneron Genetics Center.As of December 31, 2015, the Regeneron Genetics Center expanded on its foundational population-based collaboration with Geisinger Health Systems with over a dozen collaborations with academic institutions, government entities, and health systems, and had achieved the ability to sequence exomes at the rate of 100,000 per year.•Collaboration agreement with the Biomedical Advanced Research and Development Authority (BARDA)of the U.S. Department of Health and Human Services to develop, test, and manufacture a monoclonal antibody therapy for the treatment of Ebola virus infection.•Named one of the two top employersin the global biopharmaceutical industry byScience Magazinefor the fifth consecutive year, the fourth most innovative company in the world byForbes, and one ofFortune Magazine's100 best places to work.
These and other recent accomplishments have contributed to the creation of significant value for our shareholders. The Company's strong performance is reflected in the appreciation of business,9our stock price, which increased 32%, 217%, and 1554% over the one-, three-, and five-year periods ended December 31, 2015, respectively. This shareholder return places our common stock performance in the 85th, 90th, and 99th percentile, respectively, of all NASDAQ-listed companies with a market capitalization greater than $5 billion in those periods. In addition, our common stock was the 3rd, 4th, and top performing stock in our Peer Group, discussed further in this Compensation Discussion and Analysis section, over the one-, three-, and five-year periods ended December 31, 2015. See "Section 3 – Executive Compensation Process and Considerations – Peer Group" for the definition of our Peer Group and our Biotech R&D Peers.
As illustrated by the chart below, our total shareholder return ("TSR") significantly outperformed bothunderperformed the S&P 500 Index and the S&P Biotechnology Select Industry Index last year and over the past five years.
36years ended December 31, 2019, which we believe was due to factors including concerns about competitive threats to our marketed products (particularly EYLEA) and drug-pricing legislation or regulatory reform. However, over the 10-year period ended December 31, 2019, we delivered stock price appreciation of 1,453% and outperformed both the S&P 500 Index (2009–2019 TSR of 256%) and the S&P Biotechnology Select Industry Index (2009–2019 TSR of 442%). While we cannot predict or control the performance of our stock price in any particular time period, we believe that if we continue to deliver operational, pipeline, and financial results, the creation of shareholder value and stock price appreciation will follow.
Executive Compensation
Regeneron 5-Year TSR vs. S&P Indices
Indices*
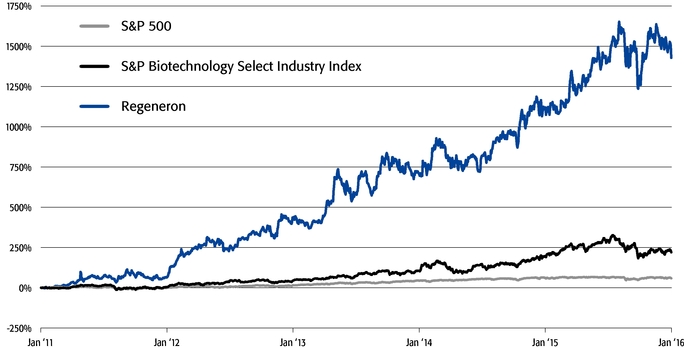

*TSR data reflect total returns (stock price appreciation and, if applicable, reinvested dividends).
Compensation Objectives and ElementsHOW OUR PAY PROGRAM WORKS
We believe that the leadership of the current executive team has been instrumental to our success in 2015 and prior years, and that an executive compensation program that attracts, motivates, and helps retain key executives, including the Named Officers, is critical to our long-term success.
Our executive compensation program is designed to achieve four main objectives:
•pay for performance;•closely align the interests of shareholders and management;
•strike a balance between short- and long-term perspectives andsupport ourlong-term growth prospects;business model and•attractdrive our product pipeline. We operate in an industry with fierce competition for talent, and believe an attractive compensation program is necessary to incentivize, motivate, and retainhighly skilled and talented executives in a competitive marketplace.
To realize these objectives, we utilize five primary compensation elements, which are summarized in the table below and discussed in detail under "Section 4 – Elements of Executive Compensation":
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
37top talent.
Executive Compensation
The Compensation Committee considered eachrelies primarily on the following compensation elements to achieve these objectives:
| Period | Element | Objective | Performance Measured/Rewarded | |
| FIXED | Annual | Base Salary | Recognizes an individual’s role and responsibilities and serves as an important retention vehicle | Reviewed annually and set based on market competitiveness, individual performance, and other internal considerations |
| PERFORMANCE - BASED | Annual | Annual Cash Incentive | Motivates and rewards our executives for short-term achievements and milestones towards our long-term goals | Corporate performance (CEO and CSO); corporate performance and individual contributions to that achievement (other NEOs) |
| Long-Term | Stock Options | Supports the achievement of strong, long-term share price growth | Options vest annually over four years; 10-year term | |
| Long-Term | PSUs (CEO and CSO)* | Aligns the interests of CEO and CSO and shareholders and serves as an important retention vehicle; rewards strong TSR performance | Five-year TSR as primary performance metric with 61% five-year TSR required to earn target | |
| Long-Term | RSAs (NEOs other than CEO and CSO) | Promotes employee retention | Annual awards vest 50% on the second anniversary of the date of grant and 50% on fourth anniversary of the date of grant |
| * | As noted above, PSUs were also granted as part of the 2019 annual equity award of the Chairman of the Board. The Chairman’s compensation is outside of the scope of this CD&A and is discussed under the subsection “Board of Directors—Compensation of Directors.” |
| 9 | Information about additional accomplishments in 2019 is provided below under the subsection “Components of Executive Pay: What We Pay and Why We Pay It – Annual Cash Incentives.” |
60 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
KEY GOVERNANCE FEATURES OF OUR COMPENSATION PROGRAM
Our Compensation Committee independently oversees the executive compensation program with the support of an independent compensation consultant and management. Our compensation program demonstrates strong governance, minimizing inappropriate risk-taking behavior while protecting shareholder rights and interests. The following is a summary of some of our executive compensation best practices and policies.
| WHAT WE DO | ||||
 | Align pay with performance |  | Maintain a strong recoupment (clawback) policy | |
 | Align management and shareholder interests |  | Review peer group pay as reference point | |
 | Maintain robust stock ownership guidelines |  | Retain an independent compensation consultant | |
 | Apply “double trigger” change-in-control vesting provisions to equity awards |  | Actively and regularly engage with shareholders on executive compensation matters | |
| WHAT WE DON’T DO | ||||
 | Reprice or exchange stock options |  | Provide excise tax gross-ups in any new compensation plans or arrangements | |
 | Provide excessive perquisites |  | Allow hedging or pledging of securities | |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 61 / 61 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
OUR COMPENSATION PHILOSOPHY AND OBJECTIVES
We consider the following objectives inwhen determining the 2015 compensation of our executives, including the Named Officers, as discussed in greater detail for each of these objectives under "Section 2 – Analysis of 2015 Executive Compensation Based on Compensation Objectives." In particular:
•We believe in performance-based compensationNEOs andlong-term incentives.In 2015, we continued to rely primarily on performance-based compensation, both for our short-term (cash bonus) and long-term incentives (stock option awards). This emphasis on performance-based compensation (particularly long-term incentives in the form of stock options) has been a consistent part of our philosophy since Regeneron's inception, including prior to the significant appreciation in Regeneron's stock price that began in early 2011.•- other executives:
1 Driving innovation through our ownership culture while managing the dilutive impact of equity grants 4 Balancing year-over-year consistency and flexibility in making compensation decisions 2 Providing at-risk, performance-based pay to all employees, with increased performance accountability as responsibility increases 5 Aligning our executive compensation with shareholder interests by linking compensation to the core elements of our long-term performance 3 Prioritizing design simplicity, long-term orientation, and avoiding too much emphasis on short-term metrics 6 Using independent sources of expertise and comparative data to inform our decision-making 7 Assessing our compensation objectives in light of measurable results 1 Driving innovation through our ownership culture while managing the dilutive impact of equity grants We
believe that time-based stock options are inherently performance based, as they provide value to employees only if there is future stock price appreciation and do not provide any value to employees if the stock price declines below the exercise price.As illustrated by the charts in "Section 2 – Analysis of 2015 Executive Compensation Based on Compensation Objectives," this emphasis on stock options has resulted in close alignment of our Chief Executive Officer's compensation in 2015 and over the last five years with the performance of our common stock over those periods:oBoth in 2015 and over the five-year period ended December 31, 2015, the year-over-year increases in our Chief Executive Officer's compensation were principally attributable to the significant appreciation in our stock price, which increased the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model for valuing stock options.oOver the same periods, the Black-Scholes grant date fair value of stock option grants to our Chief Executive Officer increased less than the appreciation of our stock price, in part because the absolute number of stock options granted to our Chief Executive Officer decreased in the last three years. The number of shares underlying the annual stock option award to our Chief Executive Officer in 2015 was approximately 39% lower than in 2012, while the stock price appreciated 217% over the same period. As a result, the appreciation in the reported value of our Chief Executive Officer's pay was significantly below the appreciation of our stock price, both cumulatively over the five-year period and on a year-over-year basis. This means that the value of our long-term shareholders' investment in Regeneron grew more rapidly than our CEO's pay over those periods.
oTo further illustrate this point, over the last five years, our Chief Executive Officer's total direct compensation, as a percentage of Regeneron's capitalization in the year in which the compensation was awarded, decreased from 0.20% to 0.08%.oAs a result of our emphasis on performance-based compensation, on a relative basis when compared to our Peer Group, the total direct compensation of our Chief Executive Officer over the last three years was also closely aligned with the performance of our common stock even when taking into account the reported grant date fair value of our Chief Executive Officer's stock option awards as determined according to the Black-Scholes model.
•We believe in year-over-year consistency in making compensation decisions and in striking a balance between the dilutive impact of equity grants and the competitiveness of our compensation program.In our compensation decisions, wefocus on the number of shares underlying equity awardsrelative to the numberas a percentage of basic shares ofcommoncapital stock outstandingrather than the grant date fair value of the award (as determined according to the Black-Scholes model).when making decisions on equity-based pay for our NEOs. We believe thisownership- anddilution-based approachto awarding stock options provides a better measure of the amount of potential increases in shareholder value that would be shared by the awards andallows us to evaluatesuchstock option and other equity grants on a consistent basisas comparedyear-over-year, without regard toother companies and regardless offluctuations in the share price, and is aligned more closely with shareholder interests. We also believe this approach allows for a more meaningful comparison to industry peers.The 2019 CEO equity grant as a percentage of
Regeneron's or other companies' common stock. Further, focusingbasic shares outstanding was below the 75th percentile of the market composite data prepared by Radford from the 2019 Radford Global Life Sciences Survey (U.S. public biotechnology and pharmaceutical companies with between 1,500 and 20,000 employees) and our Peer Group data (we refer to the composite data below as the “Market Composite Data”).10CEO Grant as a Percentage of Basic Shares Outstanding*
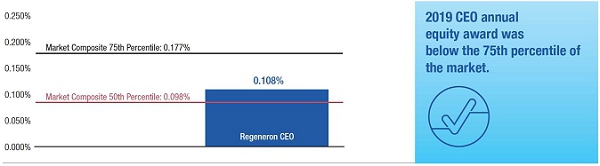
* Based on 2019 equity award information reported in the Summary Compensation Table included in this proxy statement for Regeneron and on the Market Composite Data. 10 Unless otherwise noted, award percentages in this section are based on the number of underlying shares (on an options-equivalent basis), with RSAs/RSUs and PSUs (PSUs at target) converted into options-equivalents based on a ratio of 4 to 1 and approximately 3 to 1, respectively. 62 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
Focusing on the number of shares, and the incremental sharing rate of potential future upside (ratherrather than targeting a specific Black-Scholes grant date fair value)value, avoids rewarding officersexecutives with larger grant sizes following a decline in stock price. In line with this approach, when our stock price.
oAs a percentageprice declined or remained relatively flat in each of 2016, 2017, 2018, and 2019 compared to thetotal basic shares outstanding,prior year, we continued to reduce the2015number of stockoption awardoptions awarded to ourChief Executive Officer was significantly below the 75thpercentile of the companies included in the 2015 Radford Global Life Sciences Survey and only slightly above the 50thpercentile (at 0.166% compared to 0.290% and 0.154%, respectively).CEO. Inaddition, this award was below the 50thpercentile of our Biotech R&D Peers (which was 0.183%).oIn 2015, the Compensation Committee reduced2019, the number of shares underlyingthe annual stock option awardsour CEO’s award was reduced by 10%, which contributed tothe Named Officers by 15% compared to 2014 (other than Mr. Terifay's award, which remained at the 2014 level due to his promotion to Executive Vice President, Commercial). This decrease constituted the third consecutive double-digit percentage decreasea 22% reduction in theannual grant of stock options to our Named Officers, in each case following outstanding TSR performance. In reducing the size of 2015 annual stock option awards to executives, the Compensation Committee sought to reduce the
38
Executive Compensation
oWe continued to pay close attention to our burn rate. Despite the expansive growth of our employee base, which increased by 121% between 2012 and 2015 (from 1,950 full-time employees to 4,303 full-time employees), our burn rate decreased from 5.4% to 4.4% over the same period, and we maintained a three-year burn rate average of 4.1% in 2015. We achieved this reduction through implementing three consecutive double-digit percentage decreases in the number of shares underlying annual stock option awards, without eliminating the broad-based nature of our equity compensation program.
potential dilutive impact of new equity awards without adversely affecting the competitiveness of our executive compensation program, which has successfully motivated our senior management team to deliver high operating performance and shareholder value.
oWe believe our approach to equity compensation has helped us to successfully grow and manage employee attrition, as evidenced by our 2015 employee turnover of approximately 6%, which compares favorably to the average employee turnover of approximately 18% for the life sciences sector based on the Fourth Quarter 2015 Radford Global Life Sciences Trends Report.
Highlights of Compensation Policies and Practices
We are committed to maintaining rigorous corporate governance standards, including those related to the oversight of our executive compensation policies and practices. We have compensation policies and practices designed to enhance governance of our executive compensation program and to further our compensation objectives. These policies and practices include:
|
| |
39
Executive Compensation
|
Pay for Performance
We believe in rewarding performance and establishing a strong link between compensation and both individual and corporate performance at all levels of the Company. We also believe that accountability and total compensation potential should generally increase with position and responsibility and that the proportion of the performance-based component of compensation should increase with position and responsibility. Consistent with this view, individuals with greater responsibility and ability to influence our achievement of corporate goals and strategic initiatives generally are targeted with higher levels of cash compensation, but have a higher proportion of their total cash compensation represented by cash bonus, the amount of which is based on individual and/or corporate performance and is, therefore, at risk. Similarly, equity-based compensation is higher for persons with higher levels of responsibility, making a significant portion of their total compensation dependent on long-term stock appreciation and, therefore, long-term corporate performance.
This pay-for-performance philosophy was strongly reflected in the mix of compensation elements, or "pay mix," of our Named Officers in 2015. The following charts display the mix of 2015 compensation (fixed vs. performance-based and long-term vs. short-term) of our Chief Executive Officer and our other Named Officers (excluding our Chief Executive Officer) and the mix of compensation elements of the companies included in the 2015 Radford Global Life Sciences Survey. As shown in these charts, 97% and 96% of the total direct 2015 compensation of our Chief Executive Officer and our other Named Officers, respectively, consisted of performance-based compensation, which exceeds the average percentage of performance-based compensation paid by the companies included in the 2015 Radford Global Life Sciences Survey by 7% and 14%, respectively. Accordingly, based on these metrics, our 2015 executive compensation program was more heavily weighted toward "at risk," performance-based components.
40
Executive Compensation
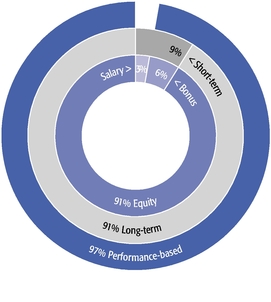 | 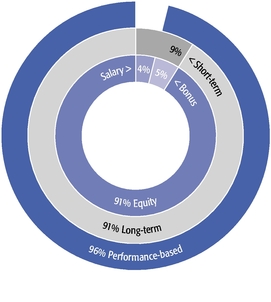 | |
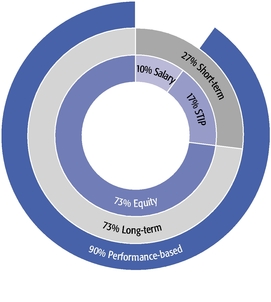 | 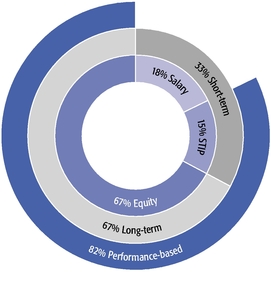 |
Based on 2015 compensation information reported in the Summary Compensation Table on page 61 for Regeneron and on the 2015 Radford Global Life Sciences Survey (comprising U.S. public biotechnology and pharmaceutical companies that have between 800 and 15,000 employees). "Equity" (shown as part of the inner circle in the charts above) reflects theaggregate grant date fair value of equity awards; and "STIP" (shown as parthis award.
CEO Annual Equity Award*
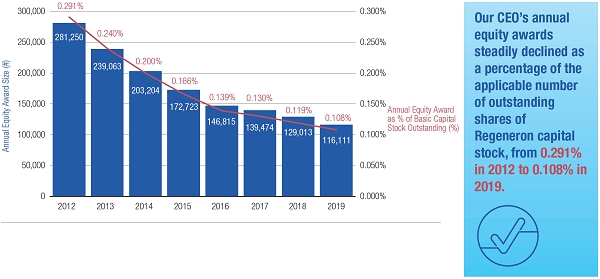
| * | Share percentages are based on 96.6 million, 99.4 million, 101.7 million, 104.1 million, 105.5 million, 107.4 million, 108.2 million and 109.8 million shares (in each case consisting of common stock and Class A stock) outstanding as of October 12, 2012, October 28, 2013, October 16, 2014, October 16, 2015, October 20, 2016, October 20, 2017, October 19, 2018, and October 24, 2019, respectively, as reported in Regeneron’s Quarterly Report on Form 10-Q for the third quarter of the applicable year. The number of shares underlying PSUs (at target) is converted into options-equivalents based on a ratio of approximately 3 to 1. | |
| 2 | Providing at-risk, performance-based pay to all employees, with increased performance accountability as responsibility increases |
All of the inner circle in the charts above) consists of bonus and/or other applicable compensation provided under short-term, non-equity incentive plans. "Long-term" compensation (shown as part of the middle circle in the charts above) consists of equity; and "short-term" compensation (shown as part of the middle circle in the charts above) consists ofour CEO’s direct pay, except for base salary, and bonus/STIP. "Performance-based"is “at-risk.” At-risk pay comprised 93% of our CEO’s direct pay in 2019 (the breakdown was similar in recent prior years). This is significantly higher than the percentage of at-risk compensation (shownfor CEOs in our Peer Group (based on the Peer Group median for each compensation component), as part of the outer circle in the charts above) consists of bonus/STIP and equity. Total compensation amounts reflect total direct compensation (total reported compensation, other than amounts reported under "All other compensation").
As shown in the charts below.

| * | “At-risk Equity” consists of stock options and any equity awards with performance-based vesting conditions (such as PSUs). “Other Equity” consists of all other equity awards, such as time-based RSAs and RSUs. “At-risk Equity” and “Other Equity” reflect the grant date fair value of such equity awards. “STIP” consists of bonus and/or other applicable compensation provided under short-term, non-equity incentive plans. “At-risk” compensation consists of STIP and At-risk Equity. Total compensation amounts reflect direct compensation (total reported compensation, other than amounts reported as “All other compensation”). |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 63 / 63 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
The “at-risk” design of our CEO’s compensation package is also demonstrated by the history of limited payouts in respect of periods with flat or negative TSR. See “Compensation-Related Matters – Introduction – Our Compensation Program Today: Aligning Pay and Performance” above for a comparison of our CEO’s reported versus realized/ realizable pay over the Compensation Committee manageslast five years.
| 3 | Prioritizing design simplicity, long-term orientation, and avoiding too much emphasis on short-term metrics |
We primarily rely on stock option grants for our NEOs’ equity-based pay because they are simple, do not deliver realizable compensation if shareholders fail to benefit from a stock price increase, are in sync with the pay mix such that a substantial portiontime required for discovery, development, and commercialization of pay is dedicatednovel therapies, and would be difficult to "at risk," performance-based compensation, with that portion to comprise a significantly greater percentage of total direct compensation“game,” unlike some pre-set short-term performance goals. In addition, in 2019 we introduced for our CEO and CSO (as well as compared to the averageChairman of the
companies included Board) PSUs with a long-term, five-year performance period (with an opportunity for an accelerated payout after four years if performance is strong) and straightforward TSR goals provided in the 2015 Radford Global Sciences Survey. The Compensation Committee believes that this mix of pay best aligns the interests of our Named Officers with those of our shareholders over time.
41an objective, fixed schedule.
Executive Compensation
Shareholder Alignment
We believe that our long-term performance and our robust pipeline are evidence of providing our NEOs and other employees with the right incentives. Tying compensation realized byto long-term, Company-wide success has enabled us to encourage decision-making that we believe is consistent with the long-term sustainability of our leadership team,Company and our reputation. The types of equity awards we have utilized support our strategy of driving value creation through innovation, our pipeline, and demand for our products.
| 4 | Balancing year-over-year consistency and flexibility in making compensation decisions |
The design and operation of our compensation plans (including our reliance on equity awards as a primary long-term employee incentive) for our NEOs have been consistent since the Company’s inception. Our decision-making process for setting NEO compensation, including the Named Officers, should show a strong relation to the value realized by our shareholders. This principle is reflected boththeir annual salaries, year-end cash incentives, and equity awards, has been similarly consistent in the pay mix referenced above and in our focus on stock options, as the value realizedrecent years. However, when warranted by the holdercircumstances, we adapt and make adjustments. Based on shareholder feedback and other relevant considerations, in 2019 we introduced PSUs as a component of the annual equity awards for our CEO and CSO and RSAs as a component of the annual equity awards for our other NEOs. See the subsection “Our Compensation Processes” below for further information regarding our compensation decision-making.
| 5 | Aligning our executive compensation with shareholder interests by linking compensation to the core elements of our long-term performance |
Our NEOs’ direct pay, except for base salary, depends on performance. We do this to align these senior executives’ at-risk pay with their levels of authority and with shareholders’ interests. In addition, our NEOs receive no value from their stock option or PSU awards if shareholders do not benefit, which results in a complete alignment of such compensation with shareholder interests. No amount of time will make a stock option ifdeliver any is dependent upon, and directly proportionate to, appreciation invalue unless the company’s stock price of our common stock. Since our inception, we have consistently structured our executive compensation based on our belief thatincreases. In addition, stock options naturally align executivesreward our NEOs for increasing shareholder value over the entire 10-year option term, which we believe is consistent with the creation of future shareholder value.drug discovery and development cycle. Similarly, any PSU vesting is determined over a long-term, five-year performance period (with an opportunity for an accelerated payout after four years if performance is strong). We also believe that the performance-based nature of equity awards such as stock options haveand PSUs is enhanced for companies like Regeneron whose stock price is directly impacted by pipeline developments. We further link our NEOs’ and other executives’ pay to their decisions that affect our performance by incorporating an assessment of the caliber of our drug-development pipeline into our compensation decisions.
64 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
| 6 | Using independent sources of expertise and comparative data to inform our decision-making |
The Compensation Committee retains its independent compensation consultant, Frederic W. Cook & Co., to perform projects at its direction and to provide professional expertise. We also use data compiled by management’s consultant, Radford. Comparative compensation data are used to review each component of our NEOs’ compensation against the Peer Group as well as their total annual compensation in relation to the Peer Group, while taking into account various factors such as the executive’s performance, past compensation history, experience, and the role in the Company’s success. We also look at survey data for companies in our area, in our industry, and in our size range to inform our compensation deliberations.
We use Peer Group data as a point of reference, but Peer Group data do not represent the only factor considered and we do not peg compensation to a specific percentile of our Peer Group. We also consider the practices of our “Biotech R&D Peers”—the 10-company sub-group of peers viewed as having businesses and drug discovery and development cultures that are most similar to Regeneron’s, with similarly-sized employee bases. See the subsection “Our Compensation Processes—Peer Data” below for additional information.
The Compensation Committee’s consultant does not perform any other services for the Company (other than those provided to the Corporate Governance and Compliance Committee with respect to non-employee director compensation matters, as discussed under “Board of Directors—Compensation of Directors” above) and has been fundamentaldetermined by the Committee to be independent.
| 7 | Assessing our compensation objectives in light of measurable results |
As a science-focused company, we believe in assessing our compensation philosophy and objectives, as they relate to our ability to attract, motivate,NEOs and retain top talent whose contributionsother employees, in light of measurable results. Those include our pipeline productivity and leadership have driven the Company's achievementsustaining an engaging employee culture that drives innovation, which we assess based on relevant metrics. For example, we review our attrition rate (which in each of corporate and strategic goals and resulted in a substantial enhancement of shareholder value. Over the one-, three-, and five-year periods ended December 31, 2015, our stock price increased 32%, 217%, and 1554%, respectively. This shareholder return places our common stock performance in the 85th, 90th, and 99th percentile,
respectively, of all NASDAQ-listed companies with a market capitalization greater than $5 billion in those periods. Our use of stock options ensures alignment of the interests of our executives, including the Named Officers, with those of Company shareholders, as our executives not only benefit from our successes, and the resulting appreciation of our stock, but are also impacted by decreases in our stock price.
The following chart compares our TSR over the last five years withwas at less than half the compensation of our Chief Executive Officer over the same period. We do not focus primarily on total direct compensation as a key metric, as it is derived in part from the reported grant date fair value of stock option awards as determined according to the Black-Scholes model. As further discussed below, in assessing stock option awards, we primarily considerindustry average) and the number of shares underlying the awards relative to the number of basic shares of common stock outstanding in order to evaluate such awards on a consistent basis as compared to other companiesregulatory approvals for our products (approvals for six products and regardless of fluctuations in the price of Regeneron's or other companies' common stock.
5-Year CEO Total Direct Compensation vs. Total Shareholder Return
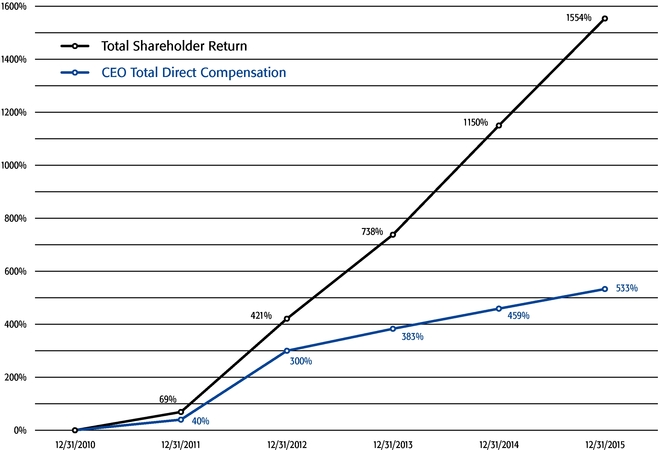
"CEOtotal direct compensation" means total reported compensation (other than amounts reported under "All other compensation").
42
Executive Compensation
As shown in the table above, compensation awarded to our Chief Executive Officer over the last five years closely tracked our TSR over the same period, and percentage increases in such compensation were considerably smaller than our TSR over the same period (533% increase in total direct compensation compared to 1554% TSR). The overwhelming portion of the increase in our Chief Executive Officer's total direct compensation over this period is directly attributable to the value of the non-cash components of his compensation package, primarily stock option awards valued according to the
Black-Scholes model. Consistent with our objective to align the interests of our executives with the interests of our shareholders, as the price of our common stock appreciated over the last five years, the value of stock option awards, the most significant component of the compensation of our Chief Executive Officer (as well as the other Named Officers), increased as well.
In addition, on a year-over-year basis, percentage increases in our Chief Executive Officer's total direct compensationeight additional key indications in the last five years were significantly below the year-over-year appreciation of10 years). We periodically review our stock price, as shown in the chart below.
Year-Over-Year Increase in CEO Total Direct Compensation vs. Total Shareholder Return
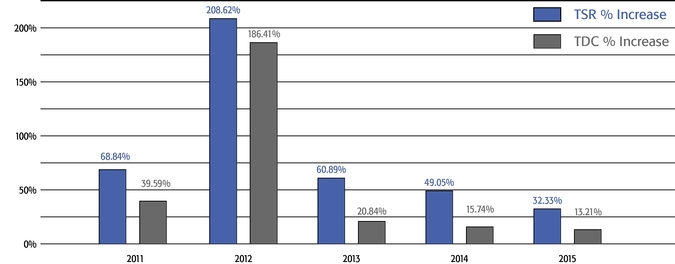
"CEO total direct compensation" means total reported compensation (other than amounts reported under "Allprogram, seek shareholder feedback, and have an open discussion about our approach to compensation and approaches utilized by other compensation").
43
Executive Compensation
The table below further demonstrates alignment of Regeneron's performance (as determined by its market capitalization) and our Chief Executive Officer's total direct compensation in the last five years. Over this period, our Chief
Executive Officer's total direct compensation, as a percentage of Regeneron's capitalization in the year in which the compensation was awarded, decreased from 0.20% to 0.08%.
CEO Total Direct Compensation as Percentage of Market Capitalization
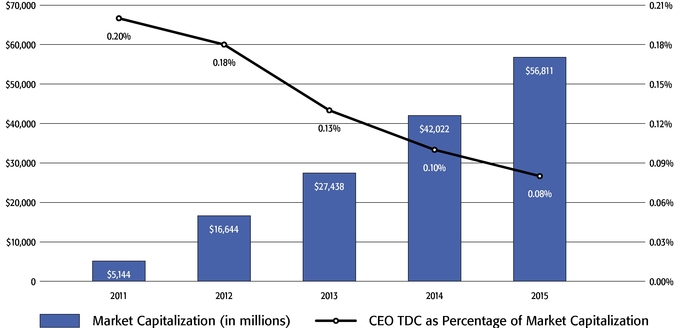
"CEO total direct compensation" means total reported compensation (other than amounts reported under "All other compensation"); and market capitalization is determined based on the number of shares of common stock and Class A stock outstanding as of December 31 of each year.
44
Executive Compensation
We further believe that the compensation awarded to our Chief Executive Officer in recent years showed a strong connection to our TSR performance over the same period both on an absolute basis and relative to the companies in our Peer Group. In addition to being the 4th best performingindustry – and make changes based on these considerations.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 65 / 65 |
company in our Peer Group over the three-year period (as shown in the chart below), we were the 3rd best and the top performing company in our Peer Group over the one- and five-year periods (in each case as measured by TSR).COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
COMPONENTS OF EXECUTIVE PAY:CEO Reported Pay Percentile versus TSR Percentile
WHAT WE PAY AND WHY WE PAY IT
OUR NEO COMPENSATION HAS FIVE PRINCIPAL COMPONENTS:

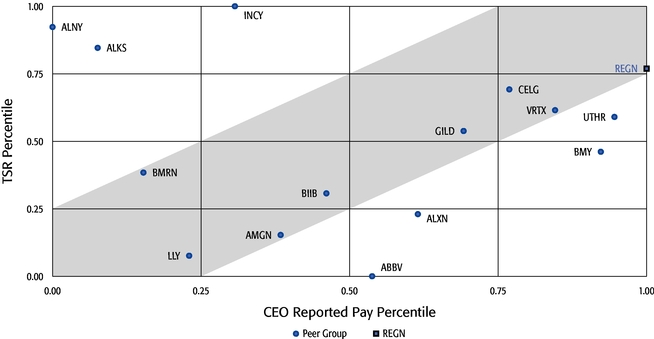
Compensation information reflects total direct compensation (i.e., total reported compensation, other than amounts reported under "Change in pension value and nonqualified deferred compensation earnings" and "All other compensation") and is based onWe use these pay components to achieve the2013 - 2015 compensation information reported in the Summary Compensation Table included in this proxy statement for Regeneron and on the data for the most recently completed three-year periods available as of December 31, 2015 for the companies included in the Peer Group. TSR information is based on 2013 - 2015 TSR for Regeneron and the Peer Group. The shaded area between the two diagonal lines indicates an alignment of pay and performance. See "Section 3 – Executive Compensation Process and Considerations – Peer Group" for more information regarding our Peer Group.following objectives:
Balance between Short- and Long-Term Perspectives
The Company's compensation program has been designed to strike a balance between short- and long-term compensation in light of the Company's available resources; its growth trajectory; its goal of offering compensation packages that are competitive with those of companies in the Peer Group and the broader biopharmaceutical industry; and its objective to
retain and motivate high-performing employees and closely align their interests with the interests of shareholders. We utilize base salary and annual cash bonuses to compensate our executives over the short term by providing a base level of income and rewarding performance on an annual basis, and we utilize stock options to foster a long-term perspective and reward long-term performance.
45
Executive Compensation
| COMPENSATION COMPONENTS | ||||||||||||||||
| Objective | Base Salaries | Annual Cash Incentives | Annual Equity Awards | Perquisites and Personal Benefits | Potential Severance Payments | |||||||||||
| Attract and retain top talent | • | • | • | • | • | |||||||||||
| Provide stability and manage risk | • | • | ||||||||||||||
| Reward annual performance | • | |||||||||||||||
|
| |||||||||||||||
| sustainable long-termperformance | • | • | ||||||||||||||
| Align our employees’ interests with those of shareholders and rewardexceptional performance | • | • | ||||||||||||||
| Promote a culture of scientificinnovation, teamwork, and ethicalbehavior | • | • | • | • | • | |||||||||||
66 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
46COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
Executive Compensation
BASE SALARIES
The base salary component of ContentsNEO pay generally comprises a steadily smaller percentage of overall compensation as executives’ level of responsibility rises.
As shown
We consider factors including the executive’s scope of responsibilities, experience, and annual performance when setting base salaries. We also consider base salaries of comparable positions in the charts under "– Payregion, among our peers, and in the broader biopharmaceutical industry. Finally, while there is no target benchmark level for Performance" above, 91%salaries, we generally position total target cash compensation (consisting of base salary and annual cash incentives) at or below the market median based on relevant comparative data. See the subsections “Our Compensation Processes—Independent Compensation Consultant” and “Our Compensation Processes—Peer Data” for further information.
A chart of our NEOs’ base salaries follows.
| 2018 Base | 2019 Base | 2019 vs. 2018 | 2020 Base | 2020 vs. 2019 | ||||||
| Named Executive Officer | Salary ($) | Salary ($) | Change (%)1 | Salary ($) | Change (%)1 | |||||
| Leonard S. Schleifer, M.D., Ph.D. | 1,330,500 | 1,377,100 | 3.5 | 1,425,300 | 3.5 | |||||
| George D. Yancopoulos, M.D., Ph.D. | 1,130,900 | 1,170,500 | 3.5 | 1,211,500 | 3.5 | |||||
| Robert E. Landry | 680,000 | 730,000 | 7.42 | 795,000 | 8.93 | |||||
| Daniel P. Van Plew | 660,000 | 683,100 | 3.5 | 795,000 | 16.43 | |||||
| Andrew J. Murphy, Ph.D. | 517,500 | 600,000 | 15.92 | 700,000 | 16.73 |
| 1 | Reflects a 3.5% merit increase consistent with those for other employees. |
| 2 | Reflects (i) a 3.5% merit increase consistent with those for other employees and (ii) a base salary adjustment of $26,200 and $64,400 to reflect promotions for Mr. Landry and Dr. Murphy, respectively. |
| 3 | Reflects (i) a 3.5% merit increase consistent with those for other employees and (ii) a base salary adjustment of $39,400, $88,000, and $79,000 to ensure greater market competitiveness for Mr. Landry, Mr. Van Plew, and Dr. Murphy, respectively. |
Our NEOs are eligible for cash incentives based on annual performance. We use these annual incentive opportunities to reward short-term achievements and milestones towards our long-term goals.
We focus on our overall corporate performance to determine the cash incentives of our CEO and our CSO. Our other NEOs’ cash incentives are assessed on both our overall corporate performance and on their individual contributions. For 2019, annual cash incentive opportunities for each NEO were weighted as shown below:
| Role | Corporate Performance | Individual Performance |
| CEO and CSO | 100% | — |
| Other NEOs | 60% | 40% |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 67 / 67 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
Corporate Performance
Feedback from our shareholder engagement efforts since our last annual meeting suggested that the annual cash incentive goals appeared subjective, and investors expressed a desire for more concrete, objective, and transparent goals so that they could more fully understand how cash incentive payments were linked to corporate and individual performance. Our Compensation Committee listened to this feedback and enhanced the existing process for determining the cash incentive for 2019. The enhanced approach also incorporates greater involvement of all non-employee directors and the Chairman of the total direct compensationBoard in reviewing the Company’s performance for purposes of setting the annual cash incentive.
Corporate performance for 2019 was assessed in three categories: (1) factors related to our product pipeline and development for both the near- and long-term; (2) factors regarding our financial performance and operations; and (3) factors related to our talent, culture, and corporate responsibility. As in prior years, the factors analyzed under category (1) continued to be a key measure for the Compensation Committee’s assessment and calculating the Company performance multiplier. This recognizes the importance of innovation as a key component of the Company’s business strategy and valuation, as well as the critical role of the development pipeline in the Company’s long-term success.
68 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
| (1) PRODUCT PIPELINE AND DEVELOPMENT (PRIMARY FACTORS) | ||
Regulatory & Clinical Milestones; Commercial Support • Approval of new products or indications by the U.S. Food and Drug Administration (“FDA”) or applicable regulatory authorities outside the United States • Regulatory submissions for new products and new indications • Breakthrough Therapy or orphan drug designations by the FDA (or their equivalent outside the United States) • Data readouts and key publications from potentially pivotal/registrational studies • Initiation of new Phase 3 or Phase 2 studies | ||
| Achievements in 2019:* | ||
EYLEA • FDA approval for diabetic retinopathy • FDA approval of pre-filled syringe;650,000 doses prepared for launch Dupixent • FDA and European Commission(“EC”) approval for adolescents(ages 12-17) with moderate-to-severeatopic dermatitis (“AD”) • Supplemental Biologics LicenseApplication (“sBLA”) and MarketingAuthorization Application (“MAA”)submissions for 300mg auto-injector;MAA approval for 200 mg auto-injector(response to FDA Complete ResponseLetter for 200 mg in progress) • FDA and EC approval for chronicrhinosinusitis with nasal polyposis(“CRSwNP”) (via priority review) • Positive results from two Phase 3 trialsin CRSwNP published inThe Lancet • EC approval for adults and adolescentswith severe asthma • Positive top-line Phase 3 results inpediatric AD (ages 6-11) • Initiated Phase 3 trial in chronicobstructive pulmonary disease • Submitted sBLA for children ≥6 to <12years with moderate to severe AD • Submitted sBLA for 2mL (300 mg)autoinjector | Libtayo • Conditional EC approval for advancedcutaneous squamous cell carcinoma(“CSCC”) • Updated positive Phase 2 data in CSCC;initiated Phase 3 adjuvant study • Continued to recruit in multipleregistrational-intent Phase 2 and Phase3 studies in cervical cancer, basal cellcarcinoma, and non-small cell lung cancer Praluent • FDA and EC approval to reduce risk ofserious cardiovascular events • FDA approval for primary hyperlipidemia(including heterozygous familialhypercholesterolemia) • Phase 3 read-out for homozygousfamilial hypercholesterolemia (“HoFH”)lipid-lowering study REGN-EB3 • Four-agent trial stopped early due toREGN-EB3 superiority to ZMapp control;full data published inNew EnglandJournal of Medicine • Breakthrough Therapy designationgranted by FDA • Rolling BLA submission for Ebolaunderway Evinacumab (ANGPTL3) • Positive Phase 3 results in HoFH Fasinumab (NGF) • Completed enrollment in Phase 3 efficacyand safety studies in osteoarthritis pain | Garetosmab (Activin A) • Completed enrollment and final visitfor double blind (pivotal) portion ofthe Phase 2 study in fibrodysplasiaossificans progressiva Other Investigational Compounds • REGN3500 (IL-33) in asthma: positivePhase 2 results • REGN1908-1909 (Feld1) in cat allergy:initiated Phase 2 study • Pozelimab (C5) in paroxysmal nocturnalhemoglobinuria: declared proof ofconcept and initiated Phase 3 program • REGN5096 (GFRa3) in osteoarthritispain: initiated Phase 2 study • REGN1979 (CD20XCD3) in non-Hodgkin lymphoma (“NHL”): positivePhase 1 results in relapsed/refractoryB-cell NHL; initiated registrational-intent Phase 2 study in relapsed/refractory follicular lymphoma;amendment to expand Phase 2 studyinto multiple other NHL subtypes ispending at sites • Narrowed development scope ofevinacumab beyond rare diseases |
| Progress in Earlier-Stage Clinical Programs; New Candidates into Clinical Development | ||
• Data readouts and key publications from existing Phase 1 studies • Initiation of new Phase 1 studies • Notable early research milestones and collaborations | ||
Achievements in 2019: | ||
• Overall, 85 clinical studies in progress(or planned through December 2019)involving 6,800 new patient volunteersin 53 countries vs. 61 clinical trials of5,600 patients in 44 countries in 2018 • REGN5458 and REGN5459(BCMAXCD3) in multiple myeloma:initiated Phase 1 studies; receivedencouraging first data from advancedmyeloma patients • REGN5713-5714-5715 (Betv1) in birchallergy: initiated Phase 1 study | • REGN5093 (METXMET) in MET-alteredadvanced non-small cell lung cancer:initiated Phase 1 study • REGN5678 (PSMAXCD28) in prostatecancer: initiated Phase 1 study • Collaboration with AlnylamPharmaceuticals, Inc. to discover anddevelop RNA interference therapeuticsfor ocular and central nervous systemdiseases; 7 targets selected | • First 50,000 exomes from UK Biobankhealth resource sequenced by theRegeneron Genetics Center® (“RGC”)and publicly released • 1,000,000 exomes sequenced by theRGC (anticipated by early 2020) • 1,694 worldwide patents issued in 2019(through December 1) • 1,376 worldwide patent applicationsfiled in 2019 (through December 1) • 83 peer-reviewed publications fromJanuary through mid-October of 2019 |
| * | Descriptions of achievements are based on information available to the Committee when setting the Company performance multiplier for 2019. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 69 / 69 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
| (2) FINANCE AND OPERATIONS (SECONDARY FACTORS) | ||
| Financial Metrics | ||
• Growth in total revenues • Growth in net product sales for key marketed products • Growth in profitability metrics | ||
| Achievements in 2019: | ||
• Total revenue for the first nine monthsof 2019 was $5.694 billion, a 19%increase over the same period in 2018($4.783 billion) • Full-year Non-GAAP Net Incomeand Diluted Non-GAAP EPS forecastexceeded the 2019 Board-approvedplan by double digits • Double-digit free cash flow growth (netcash provided by operating activitiesless capital expenditures) for the firstnine months of 2019 versus the sameperiod in 2018 | • EYLEA global net product sales of$5.537 billion in the first nine months of2019 (representing 12% growth vs. thefirst nine months of 2018) • EYLEA net product sales in the U.S.increased on an annual basis for theeighth straight year without a priceincrease • Dupixent global net product sales of$1.564 billion in the first nine months of2019 (representing 159% growth vs. thefirst nine months of 2018) • Praluent global net product sales of$207 million in the first nine months of2019 (representing 3% decline vs. thefirst nine months of 2018) | • Kevzara global net product sales of$147 million in the first nine months of2019 (representing 139% growth vs. thefirst nine months of 2018) • Libtayo global net product sales of$119 million in the first nine months of2019 • Initiated a share repurchase programto repurchase up to $1.0 billion ofcommon stock |
| Operational & Manufacturing | ||
• Marketing structure & strategy • Pricing, policy & legal developments • Successful completion of global audits • Expansion of facilities • Manufacturing volume | ||
Achievements in 2019: | ||
• Expanded and realigned EYLEA field force to focus on realizing greater opportunity in diabetic eye disease • Executed well-received Dupixent DTC TV U.S. campaigns for adult AD and asthma • Implemented new pricing strategy for Praluent, reducing U.S. list price • U.S. District Court invalidated Amgen Inc.’s asserted patent claims targeting PCSK9 | •U.S. and EU patent offices invalidatedImmunex Corporation’s patentsclaiming IL-4R antibodies •U.S. District Court found that themanufacture, use, offer for sale, sale,or importation into the United States ofaflibercept does not infringe NovartisAG’s asserted patent claims •Continued work with policymakersto develop responsible solutions thataddress affordability and accessibilitywhile preserving incentives to developtransformative treatments of the future | • IOPS facility in Raheen fullyoperational; largest biotech facility inIreland • IOPS completed 7 global successfulinspections and partner audits in 2019,including FDA, U.S. Department ofAgriculture, and partner audits |
70 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
| (3) TALENT, CULTURE, AND CORPORATE RESPONSIBILITY | |||
| Talent Management & Retention | |||
• Growth of global workforce to support our long-term strategic objectives • Employee retention and below-industry attrition rate • Outside recognition and employee feedback | |||
| Achievements in 2019: | |||
• Hired ~2,000 individuals to workforce,including nearly 1,200 full-timeemployees from January 1, 2019 toNovember 1, 2019; workforce to over8,000 full-time employees as of the endof that period • Continued to maintain a rolling12-month turnover rate of ~7.5%; lessthan half the industry average of 19%(based on Radford’s report for thelife sciences industry for the secondquarter of 2019) | • Rolled outThe Regeneron Way,Regeneron’s updated cultural valuesand behaviors that define who we are,what we stand for, and how we work together • Science “Top Employers” - #2company to work for in the globalbiopharmaceutical industry (ranked#1 or #2 for past nine years, makingRegeneron the most highly rankedcompany over the past decade) • Fortune “100 Best Companies to WorkFor” | • Forbes“Best Employers for Diversity” • Forbes“World’s Best Employers” and“America’s Best Employers by State” • Fortune/Great Places to Work “BestPlaces to Work in Health Care andBiopharma” • Great Places to Work “Best Workplacesin Ireland” • Fast Company “Best Workplaces forInnovators” | |
| Corporate Responsibility | |||
• ESG activities, reporting, ratings, and rankings • Corporate giving • Philanthropic and citizenship programs | |||
| Achievements in 2019: | |||
• Published second annualResponsibility Report, expandeddisclosure on relevant ESG issues, andimproved performance on several ESGinvestor assessments • Identified 2025 corporate responsibilitygoals, including broader environmentalpriorities • Included in the Dow JonesSustainability World Index, one of onlyfour biotechnology companies on thelist | • Recognized byForbes andJust Capitalas one of “America’s Most JUST Companies,” ranking of the top 100companies that are doing right by alltheir stakeholders while generatingprofits for shareholders • Included in the inauguralNewsweeklist of “America’s Most Responsible Companies” (#25) • Recognized for third time onCivic50 list of most community-mindedcompanies in the U.S. | • Over 50% employee participation inthird annualDay for Doing Good globalday of service, more than double theaverage participation rate at othercompanies • Ongoing $100 million commitment tothe Regeneron Science Talent Search • December 2019 launch of theRegeneron DNA Learning Center inconjunction with Cold Spring HarborLaboratory | |
| Corporate Reputation and Recognition | |||
• Company, leadership, and individual recognitions • Other external honors | |||
Achievements in 2019: | |||
• Forbes“World’s Most InnovativeCompanies” (#16) • Shingo Institute “The Shingo Prize” foroperational excellence • IDEA Pharma “PharmaceuticalInnovation Index” (#7) • Genetic Engineering & BiotechnologyNews “Top 25 Biotech Companies of2019” (#10) | • Optometric Center of New York “Eye onInnovation” award • Forbes“America’s Most InnovativeLeaders” (#20, Drs. Schleifer and Yancopoulos) • Harvard Business Review “Best-Performing CEOs in the World”(#93, Dr. Schleifer) • Cornell Entrepreneur of the Year(Dr. Schleifer) | • Columbia College Alexander HamiltonMedal (Dr. Yancopoulos) • Institutional Investor “Best BiotechCEOs & CFOs” (Dr. Schleifer andMr. Landry) | |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 71 / 71 |
COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS
For each of the factors listed in the table above, the Compensation Committee assessed actual performance for the year. Based on a holistic assessment of these factors, the Compensation Committee set the Company performance multiplier at 1.85, from a possible range of 0 to 2.0.
Individual Performance
The personal performance multiplier may range from 0 to 1.5. For the explanation of individual factors considered in the cash incentive decisions, see the subsection “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives.”
2019 Earned Cash Incentives
In determining the level of 2019 cash incentives earned, we calculated the NEOs’ respective target cash incentive amounts (which, for our CEO, was set approximately at the median of the Peer Group) and applied the Company performance multiplier based on the Company’s achievement of 2019 objectives; and, for the three NEOs who also have a personal performance component, we applied a personal performance multiplier. For the three NEOs with a personal performance component, the personal performance component had a 40% weighting and the Company performance component had a 60% weighting.
Based on the achievement of corporate goals (discussed above) and individual goals (discussed in the subsection “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives”) in the past year, our NEOs earned the followed cash incentives in 2019:
| Named Executive Officer | 2019 Base Salary ($) | Cash Incentive Target (as percentage of base salary) | Personal Performance Multiplier | Company Performance Multiplier | Total Cash Incentive ($) | |||||||
| Leonard S. Schleifer, M.D., Ph.D. | 1,377,100 | 120% | n/a | 1.85 | 3,057,162 | |||||||
| George D. Yancopoulos, M.D., Ph.D. | 1,170,500 | 120% | n/a | 1.85 | 2,598,510 | |||||||
| Robert E. Landry | 730,000 | 65% | 1.5 | 1.85 | 811,395 | |||||||
| Daniel P. Van Plew | 683,100 | 65% | 1.5 | 1.85 | 759,266 | |||||||
| Andrew J. Murphy, Ph.D. | 600,000 | 65% | 1.5 | 1.85 | 666,900 | |||||||
72 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
The majority of our Chief Executive OfficerNEOs’ pay is designed to reward delivery of sustainable long-term value creation, which we believe is created by focusing on the discovery, development, and commercialization of new medicines. Our equity compensation program is intended to reward this long-term performance simply, using primarily stock options and, in the case of our CEO and CSO, also PSUs and giving effect to the following considerations:

| Performance-Based Equity | ||
| • | Our NEOs’ stock option grants, which in 2019 comprised 70% of each NEO’s annual equityawards, are performance-based because we determine the award size after taking intoaccount corporate and/or individual performance assessments, and then the award onlydelivers value if we deliver stock price appreciation for shareholders after grant. | |
| • | In addition, our CEO’s and CSO’s PSU grants, which in 2019 comprised the other 30% of eachsuch executive’s annual equity award, are performance-based because we determine theaward size after taking into account corporate performance assessments, and then the awardonly vests if the performance criteria are satisfied (minimum threshold payout requires at leasta 28% TSR over five years). | |
| Long-Term Value Creation | ||
| • | Our NEOs’ and other employees’ stock option grants have ten-year terms and four-yearvesting provisions (generally requiring our employees, including our NEOs, to remainemployed for four years in order for all options to vest*) to align with long-term value creationand the development cycle of our products. | |
| • | Our NEOs’ and other employees’ RSAs/RSUs also promote long-term employment: RSAsawarded as a component of annual equity awards vest 50% on the second anniversary of thedate of grant and 50% on fourth anniversary of the date of grant, which is a more backloadedvesting schedule than is typical in the industry. | |
| • | Our CEO’s and CSO’s PSU grants incorporate a long-term, five-year performance period (withan opportunity for an accelerated payout after four years if performance is strong). | |
| Meaningful Holding Requirements | ||
| • | We require NEOs to retain a significant amount of equity within five years of their employmentwith Regeneron: | |
| • | Our CEO and CSO must own shares with a value at least 6-times their respective base salaries. | |
| • | Our other NEOs must own shares with a value at least 2-times their respective base salaries. | |
| Risk Mitigating Design Features | ||
| • | We have a recoupment (clawback) policy that enables us to reduce or recoup equity and otherincentive compensation for compliance violations by NEOs and other covered officers andemployees; importantly, the policy covers both financial and non-financial violations. | |
| • | We prohibit our NEOs from hedging or pledging Regeneron securities they hold, includingthose acquired through employee equity awards. | |
| * | In the case of our CEO, this is subject to the terms of his employment agreement. See the subsection “Compensation Dashboard—2019 Compensation Tables—Post-Employment Compensation—Leonard S. Schleifer, M.D., Ph.D. Employment Agreement.” In the case of our CSO, any unvested stock options granted to him in December 2019 will continue to vest following his qualified retirement (as defined in the applicable Company policy). |
As outlined in the chart below, our Compensation Committee utilizes a customized framework for determining the size of the annual equity awards of our NEOs and other executives. The Compensation Committee’s primary consideration is the number of shares underlying the equity awards as a percentage of basic shares of capital stock outstanding, which is evaluated on an options-equivalent basis to allow for a meaningful year-over-year comparison.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 73 / 73 |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
| PRIMARY CONSIDERATION | Targeted Percentage of Total Basic Shares Outstanding | |
| • | Our evaluation of the awards as a percentage of the total basic shares outstanding comparedto our Peer Group and the Market Composite Data. This enables us to evaluate grants on aconsistent basis regardless of stock price fluctuations of our or our reference companies’stock prices. Focusing on the number of shares also avoids an outcome where NEOs areprovided larger grants following stock price declines or penalizing high performance withsmaller grants. For our CEO, we generally target between the 50th and 75th percentiles of theMarket Composite Data. | |
| SECONDARY CONSIDERATIONS | Corporate Performance Assessment | |
| • | Our assessment of Regeneron’s corporate accomplishments for 2019, particularly as theyrelate to our product pipeline. | |
| Individual Performance Assessment | ||
| • | Our assessment of performance against the corporate goals established by the board of directorsfor the CEO and the goals established by the Committee and/or the CEO for the other NEOs, aswell as an assessment of the individual’s importance to the Company’s future performance. | |
| NEO Grant History and Retention Considerations | ||
| • | Our evaluation of each NEO’s grant history and prior grant size amounts. For retentionpurposes, the Committee also assesses the amount of outstanding unvested and/or vested butunexercised stock options and other equity awards (if applicable) held, including whether suchawards are “in-the-money.” | |
In applying this framework, the number of shares underlying the annual equity awards (on an options-equivalent basis) granted in 2019 to our CEO and CSO (as well as our other Named Officers in 2015 consisted of long-term compensation, which exceeds the corresponding average percentage for the companies included in the 2015 Radford Global Life Sciences Survey by 18% and 24%, respectively. Based on these metrics, our compensation structure is more heavily weighted toward long-term components versus short-term pay, thus promoting a long-term perspective among our executives.
Despite the reductions in theemployees, generally) was set 10% lower compared to 2018. The 2019 annual grant of stock optionsawards to our Named Officersother NEOs are described above,in greater detail below. With respect to our CEO and CSO, the grant date fair value (as determined accordingof their 2019 annual equity awards was nearly 22% lower than in 2018.
| Named Executive Officer | Annual Equity Award1 (Options-Equivalents) | Breakdown of Annual Equity Award | Year-over-Year Change in Shares Underlying Annual Equity Award | Year-over-Year Change in Grant Date Fair Value | Change in Shares Underlying Annual Equity Award Compared to 20122 | |||||||||
| Leonard S. Schleifer, M.D., Ph.D. | 116,111 | 81,278 Stock Options (70%) 11,180 Target PSUs (30%) | -10% | -21.8% | -58.7% | |||||||||
| George D. Yancopoulos, M.D., Ph.D. | 116,111 | 81,278 Stock Options (70%) 11,180 Target PSUs (30%) | -10% | -21.8% | -51.4% | |||||||||
| Robert E. Landry | 35,000 | 3 | 24,500 Stock Options (70%) 2,625 RSAs (30%) | 75% | 3 | 36.2% | 3 | N/A | 4 | |||||
| Daniel P. Van Plew | 35,000 | 5 | 24,500 Stock Options (70%) 2,625 RSAs (30%) | -24.3% | 5 | -41.1% | 5 | -53.3% | ||||||
| Andrew J. Murphy, Ph.D. | 35,000 | 6 | 24,500 Stock Options (70%) 2,625 RSAs (30%) | 40% | 6 | 8.9% | 6 | 12.5% | ||||||
| 1 | Aggregate number representing, on an options-equivalent basis, awards of stock options, PSUs, and/or RSAs. See “Compensation Dashboard – 2019 Executive Compensation Tables – 2019 Grants of Plan-Based Awards” for more information on the terms of these awards. |
| 2 | For comparison purposes, this column shows, on an options-equivalent basis, a percentage change over the period in which we reduced, on a year-over-year basis, the number of shares underlying equity awards to our CEO as well as per employee. |
| 3 | Mr. Landry’s 2019 annual equity award reflects his promotion to Executive Vice President effective January 1, 2019. Mr. Landry also received a special RSA in 2019, which is not reflected in the table above and is separately reported in “Compensation Dashboard – 2019 Executive Compensation Tables – 2019 Grants of Plan-Based Awards.” |
| 4 | Mr. Landry joined the Company in 2013. |
| 5 | Mr. Van Plew’s 2019 annual equity award was adjusted to better align with the annual equity awards of other senior executives. Mr. Van Plew also received a special RSA in 2019, which is not reflected in the table above and is separately reported in “Compensation Dashboard – 2019 Executive Compensation Tables – 2019 Grants of Plan-Based Awards.” |
| 6 | Dr. Murphy’s 2019 annual equity award reflects his promotion to Executive Vice President effective January 1, 2019. Dr. Murphy also received a special RSA in 2019, which is not reflected in the table above and is separately reported in “Compensation Dashboard – 2019 Executive Compensation Tables –2019 Grants of Plan-Based Awards.” |
74 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
Annual Stock Option Awards
As in years past, stock options remained the Black-Scholes model for valuing stock options)principal component of the annual equity awards granted to our NEOs in 2019 (comprising 70% of each NEO’s award). We have used stock options granted in 2015such a prominent manner because they are inherently performance-based, requiring stock price appreciation before there is higher thanany real value earned, and simple. No amount of time will make a stock option deliver any value unless the Black-Scholes grant date fair value of the larger number ofcompany’s stock price increases. In addition, stock options granted to Named Officers in prior years. This increase inreward our NEOs for increasing shareholder value over the Black-Scholes valueentire 10-year option term, which we believe is consistent with the drug discovery and development cycle.
The 2019 stock options all have an exercise price of annual stock option awards (and the corresponding increase in the value of total compensation for the Named Officers in 2015) is attributable largely to the higher market value of our common stock on December 16, 2015, the date of these option grants, compared to prior years. On December 16, 2015,$372.46 per share, the average of the high and low sales price per share of our common stock as quoted on the NASDAQ Global Select Market (used for calculatingon the grant date fair value) was $555.67,of grant. All of these grants consist of non-qualified stock options, which vest ratably over a period of four years. Except as compared to $399.66 on December 31, 2014set forth below in the subsection “Compensation Dashboard—2019 Compensation Tables—Post-Employment Compensation,” stock option vesting ceases, and $270.43 on December 13, 2013, the respective 2014 and 2013 grant dates.unvested stock options are forfeited, upon termination of employment.
Annual PSUs
In line with the goal of maintaining year-over-year consistency in making compensation decisions (regardless of stock price fluctuations), the Compensation Committee believes that the grant date fair value2019, we introduced PSUs as a secondary component of the optionannual equity awards for our CEO and CSO (30% of each such executive’s annual equity award (as determined accordingon an options-equivalent basis). The PSUs are performance-based because they will not vest and will not deliver realizable compensation if shareholders fail to benefit from a meaningful increase in TSR over the Black-Scholes model for valuing stock options) is notlong-term performance period.
The PSUs have a primary performance period of five years from the most appropriate measure for evaluating thedate of grant. Rather, the Compensation Committee focuses more on the percentage of Regeneron's potential future share price appreciation that is shared by meansBetween 50% and 225% of the award with the executive, expressed as thetarget number of shares underlying the option award relativePSUs granted to the numbereach executive (i.e.,between 50% and 225% of outstanding shares.
The following chart shows the decrease11,180 PSUs) may vest upon achievement of predetermined, cumulative TSR goals that were derived from compound annual growth rates of 5% to 15%, as set forth in the 2012 - 2015 annual stock option awards to our Chief Executive Officer both based on the number of shares underlying the awards and as
a percentage of the applicable number of outstanding shares of Regeneron common and Class A stock:schedule below:
| Performance Level | 5-year Cumulative TSR Goal | Payout1 |
| Maximum | +101% | 225% |
| +93% | 200% | |
| +84% | 175% | |
| +76% | 150% | |
| +69% | 125% | |
| Target | +61% | 100% |
| +44% | 75% | |
| Threshold | +28% | 50% |
| 1 | Payouts are expressed as a percentage of the target number of PSUs awarded to each executive. |
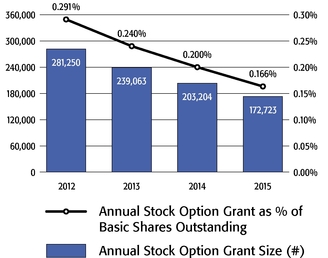
Share percentages are based on 96.6 million, 99.4 million, 101.7 million, and 104.1 million of shares (in each case consisting of common stock and Class A stock) outstanding as of October 12, 2012, October 28, 2013, October 16, 2014, and October 16, 2015, respectively, as reported in Regeneron's Quarterly Report on Form 10-Q for the third quarter of the applicable year.
In recent years, we have experienced significant growth in employees to support our research & development and commercialization efforts. From 2012 to 2015, we increased our employee base by approximately 121%. While the number of recipients of stock awards continued to increase given our practice of making initial stock option awards to all new employees and annual stock option awards to eligible employees whose performance is determined to merit an annual grant, we managed our utilization of stock awards judiciously, reducing our burn rate from the 2012 level and achieving a three-year burn rate average of 4.1% in 2015, in line with our current burn rate goal of 4%. We achieved this reduction through implementing three consecutive double-digit percentage decreases in the number of shares underlying annual stock option awards, without eliminating the broad-based nature of our equity compensation program.
47
Executive Compensation
Regeneron Stock Utilization vs. Headcount
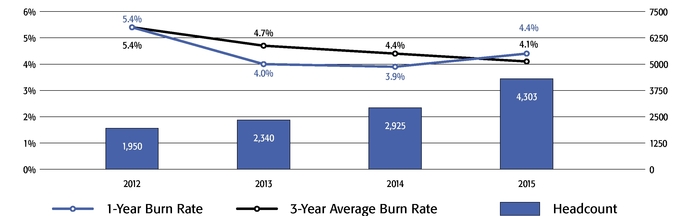
Burn rate calculated by dividing the number of shares subject to equity awards (time-based and performance-based stock options and restricted stock) granted during the year by the weighted-average number of shares of common stock (including unvested restricted stock) and Class A stock outstanding during the year. A multiplier of 2 is applied to restricted stock awards. Headcount numbers based on the number of employees as of December 31 of the applicable year.
In addition, the terms of the PSU awards provide the recipient an opportunity for accelerated vesting after four years if performance is strong, as measured against four-year cumulative TSR goals derived from the same compound annual growth rates used to calculate the cumulative TSR goals for the five-year performance period.
If no PSUs have vested at the end of the five-year performance period as a result of not having achieved the threshold cumulative TSR goals, the recipient will have an opportunity to earn a payout at threshold (50% of the target number of PSUs) if the Company’s cumulative TSR over such five-year period exceeds, on a relative basis, the cumulative TSR of the Nasdaq Biotech Index (composite return) by at least 200 basis points. The rationale for the minimum payout for exceeding the industry five-year index is that there may be circumstances in which Regeneron outperforms the broader market without delivering a positive return, such as in the last three years whencase of a recession or industry-wide developments outside management’s control.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 75 / 75 |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
Annual RSAs
In 2019, we implementedintroduced time-based RSAs as a secondary component of the stock option award reductions described above, we managed eitherannual equity awards for our NEOs other than our CEO and CSO as part of the Company-wide decision to decrease our burn rate orintroduce an RSA/RSU component to keep the percentage increaseregular long-term incentive mix for broad-based employees. RSAs comprise 30% of participating NEOs awards on an options-equivalent basis. We believe diversifying the mix of equity awards in our burn rate significantly belowthis manner responds to shareholder feedback about dilution (burn rate) and retention, as well as employee input. Each such RSA vests 50% on the percentage increasesecond anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, which is a more backloaded vesting schedule than is typical in the industry.
Special RSAs to Certain NEOs
We supplemented our annual equity awards in 2019 with highly targeted grants of time-based RSAs/RSUs to certain key employees (including three of our NEOs) to further promote employee retention and reward exceptional performance. Based on this determination, in December 2019 Mr. Landry and Mr. Van Plew each received 5,000 RSAs and Dr. Murphy received 10,000 RSAs. Each such RSA vests on the fourth anniversary of the date of grant to promote long-term employment.
PERQUISITES AND PERSONAL BENEFITS
Similar to other employees, our NEOs may participate in Company-wide health, disability, life insurance, and other benefit plans, as well as our 401(k) Savings Plan. See details concerning the 401(k) Savings Plan in the subsection “Compensation Dashboard—Additional Compensation Information—Perquisites and Personal Benefits.” Our NEOs are eligible to receive a limited number of additional perquisites. These include financial and tax planning assistance, which are taxable benefits.
In addition, our employees. The 2015 increaseCEO is entitled to life insurance, long-term disability, medical malpractice insurance premiums, and additional tax and financial planning services pursuant to his employment agreement. These are described in our burn rate comparedfootnote 4 to the prior yearSummary Compensation Table.
Our CEO and CSO are also eligible for various benefits under our Company security policy, which was approved by the board of directors in 2015 for the purpose of ensuring increased efficiencies and providing a more secure environment for these executives. Based on the recommendation of an independent, third-party security study, our security policy and related guidelines require our CEO and CSO (as well as their spouses and children when they accompany them) to use, as much as practicable, Company-provided aircraft for all business and personal air travel.
Additional information regarding perquisites and other personal benefits provided to our NEOs in, or with respect to, 2019 is wholly attributable to the rapid hiring of new key employees (as evidenced by a 47% increasegiven in the number of employees over the same period), not increased sharing with our Named Officers (whose stock options grants declined).
Regeneron Year-over-Year Change inBurn Rate and Headcount
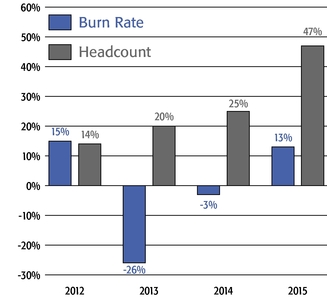
Burn rate and headcount calculated as noted above.
Market Competitiveness and Employee Retention
In determining the appropriate size of stock option awardsapplicable footnotes to executives, including the Named Officers, the Compensation Committee primarily considers the number of shares underlying the awards relative to the number of basic shares of common stock outstanding and not the grant date fair value of the award (as determined according to the Black-Scholes model). The Compensation Committee is therefore able to evaluate such grants on a consistent basis as compared to other companies and regardless of fluctuations in the price of Regeneron's or other companies' common stock. Further, focusing on the number of shares and the incremental sharing rate of potential future upside (rather than targeting a specific Black-Scholes grant date fair value) avoids rewarding officers with larger grant sizes following a decline in our stock price. The following chart displays the 2015 annual stock option award to our Chief Executive Officer as a percentage of the total basic shares outstanding, as compared to the 75th percentile and the 50th percentile of the companies included in the 2015 Radford Global Life Sciences Survey, showing that the size of his 2015 annual award was significantly below the 75th percentile and only slightly above the 50th percentile. It also shows that this award was below the 50th percentile of our Biotech R&D Peers.
48
Executive Compensation
CEO Grant as Percentage of Basic Shares Outstanding
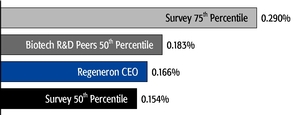
Based on 2015 stock option award information reported inthe Summary Compensation Tableincluded in this proxy statement for Regeneron; data relating to grants of equity awards from the 2015 Radford Global Life Sciences Survey (comprising U.S. public biotechnologyandpharmaceutical companies that have between 800 and 15,000 employees); and 2014 information available for Regeneron's Biotech R&D Peers (as defined in "Section 3 – Executive Compensation Process and Considerations – Peer Group"). The share information is based on 104.1 million shares (consisting of common stock and Class A stock) of Regeneron outstanding as of October 16, 2015 (as reported in Regeneron's Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2015) and on the number of shares outstanding reportedin the2015 Radford Global Life Sciences Survey for each of the companies included in the survey.
We believe that the structure offollowing three points are key to understanding our executive compensation program encourages innovation, attracts talent with entrepreneurial spirit, increases employee engagement, and improves employee retention and the continuity of executive leadership during a critical period of growth. We regard our stock option grants as a key employee and executive retention tool. As stock option awards to our employees vest over time, generally over four years, they provide an incentive for the employee to continue to contribute to our success. Historically,
stock options have helped us maintain a motivational level of total compensation for our Named Officerschange-in-control and other eligible employees, while conserving the Company's cash. We believe that this has helped us to successfully grow and manage employee attrition, as evidenced by our 2015 employee turnover of approximately 6%, which compares favorably to the average employee turnover of approximately 18% for the life sciences sector based on the Fourth Quarter 2015 Radford Global Life Sciences Trends Report. In addition, stock options have allowed us to attract and retain entrepreneurial employees and foster an ownership culture. Moreover, granting stock options as long-term incentives to executives is standard practice in our industry and is an important part of our effort to attract, retain, and motivate high-quality talent.severance provisions:
In light of these considerations, we continued our practice of annual stock option grants in 2015, although we again (for the third consecutive year) reduced the number of shares underlying the annual stock option awards to the Named Officers as described above to reduce the potential dilutive impact of executive awards without adversely affecting the effectiveness of our executive compensation program, which has successfully motivated our senior management team to deliver high operating performance and shareholder value. In light of the fierce competition for talent in our industry, we believe that it is important to continue to motivate our existing employees, including the Named Officers, through stock option awards and to ensure that they share in the success of Regeneron.
We provide our Named Officers with a limited number of perquisites and other personal benefits. These benefits, which are described in greater detail under "Section 4 – Elements of Executive Compensation – Perquisites and Other Personal Benefits" below, are periodically reviewed by the Compensation Committee.
| • | Outstanding equity award agreements (with the exceptions and qualifications described in the subsection “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments”) for all employees other than Dr. Vagelos include a governance best-practice “double trigger” provision for the acceleration of vesting of awards granted thereunder only upon a without-cause termination by the Company within two years of a change in control. |
| • | We have apolicy against excise tax gross-up provisionsfor payments contingent on a change in control of Regeneron in contracts, compensatory plans, and other arrangement with the Company’s officers (including NEOs) with the exception of the CEO under his existing employment agreement or amendments to it. |
| • | Regeneron hasno pension, deferred compensation, or retirement plansother than our 401(k) Savings Plan described above. |
For additional details, see the subsection “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments.”
 | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
OverviewOUR COMPENSATION PROCESSES
The Compensation Committee is responsible for overseeing the Company'sCompany’s general compensation objectives and programs. The Compensation Committee evaluates the performance of our Named OfficersNEOs and approves compensation for the Named Officers (intheir compensation—in the case of the Chief Executive Officer,CEO, subject to first obtaining the approval of the non-employee members of the board of directors).directors. The Compensation Committee operates under a written charter adopted by the board of directors and regularly reviews and reassesses the adequacy of its charter. A copy of the current charter is available on our website atwww.regeneron.comunder the "Corporate Governance"“Corporate Governance” heading on the "Investors“Investors & Media"Media” page.
Members of our senior management play a significant role in the overall executive compensation process and assess performance of other officers. They also recommend, for Compensation Committee approval, salary, bonus, and stock option grant budgets for non-officers and make specific recommendations for salary increases, bonuses, and equity award grants for other officers. For our Named Officers (other than our Chief Executive Officer), recommendations to the Compensation Committee regarding their compensation are made by, or with the approval of, our Chief Executive Officer, who also evaluates their performance. Our Chief Executive Officer's performance is evaluated directly by the Compensation Committee based on our overall corporate performance
49
Executive Compensation
against annual goals that are approved by the board of directors at the beginning of each year, as discussed in more detail below.
The Compensation Committee has the sole authority to retain, at our expense, one or more third-party compensation consultants to assist the Compensation Committee in performing its responsibilities and to terminate the services of the consultant if the Compensation Committee deems it appropriate. In 2015, the Compensation Committee utilized the services of Frederic W. Cook & Co. to assist it in fulfilling its responsibilities. In order to maintain its independence, Frederic W. Cook & Co. was retained directly by the Compensation Committee and performed projects at the Compensation Committee's direction. The Compensation Committee's consultant reviews management recommendations on compensation plans, budgets, and strategies and advises the Compensation Committee on regulations and trends in executive compensation nationally and specifically in the pharmaceutical and biopharmaceutical industries. The Compensation Committee's consultant provides comparative compensation information for our Chief Executive Officer and other senior executives (using the Peer Group and other compensation data as described below), reviews senior management's compensation recommendations for other officers, including the other Named Officers, and provides general advice to the Compensation Committee on compensation matters, including facilitating the articulation and periodic review of the Company's compensation philosophy.
Annual salaries for the following year and year-end bonusescash incentives and stock option awards or other year-end equity awards for all employees are determined in December of each year based on Company and individual performance, as well as other factors, includingwhich may include compensation trends among our Peer Group and in the biotechnology industry in general. The 2015 salaries and 2014 year-end bonuses and stock option awards for our Named Officers were established by the Compensation Committee in December 2014. In November and December 2015, the Compensation Committee reviewed the performance of each of the Named Officers and presented its recommendations for 2016 salaries and 2015 year-end bonuses and equity awards for the Named Officers to the non-employee members of the board of directors for concurrence. With respect to our Chief Executive Officer,CEO, this process is formalized in the charter of the Compensation Committee,Committee’s charter, which specifies that the Compensation Committee is to annually present the proposed annual compensation of the Chief Executive OfficerCEO to the non-employee members of the board of directors for approval.
For purposes of setting compensation of our Chief Executive Officer, our Chief Scientific Officer, the other Named Officers, and other senior executives, we use comparative compensation information from a relevant peer group of companies ("Peer Group"). The companies in the Peer Group are selected by the Compensation Committee, with the assistance of Frederic W. Cook & Co., based on factors including, but not limited to, market capitalization, geographic location, number of employees, therapeutic focus, research and development expenditures, stage of development, total revenues, and product sales. The Company's trailing revenue size, number of employees, operating earnings, and market capitalization are all nearly in the middle of the range of the current Peer Group companies. The Peer Group is also meant to provide a representative sample of companies with which we compete for talent. The Compensation Committee periodically reassesses the composition of the companies within the Peer Group and makes changes as appropriate, taking into account factors such as changes in the Company's market capitalization and merger-and-acquisition activity impacting the existing Peer Group companies. In September 2015, the Compensation Committee approved a new Peer Group based on the recommendation of its compensation consultant, Frederic W. Cook & Co.. The changes to the Peer Group were as follows: (i) elimination of Cubist Pharmaceuticals, Inc. in light of the fact that it had been acquired by Merck & Co., Inc.; (ii) elimination of Seattle Genetics, Inc. due to its market capitalization being significantly below Regeneron's; and (iii) addition of Alkermes plc and Alnylam Pharmaceuticals, Inc., both of which are biopharmaceutical companies with a business model the Compensation Committee considered similar to Regeneron's. In approving the new Peer Group, the Compensation Committee also took into account that, as of the approval date, Regeneron was the median company in the Peer Group based on market capitalization, revenues for the last four completed quarters,non-employee directors and the then-available reported number of employees, as shown in the table below.
50
Executive Compensation
Market Capitalization (in millions) | Last Four Quarter | | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
As of 8/31/2015 | 12-Month Average | Revenue (in millions) | Employees* | ||||||||||||||||
Gilead | $ | 154,201 | Gilead | $ | 157,984 | Gilead | $ | 29,194 | Lilly | | 39,135 | ||||||||
Amgen | $ | 115,087 | Amgen | $ | 119,866 | AbbVie | $ | 20,986 | AbbVie | | 28,000 | ||||||||
AbbVie | $ | 103,306 | AbbVie | $ | 102,021 | Amgen | $ | 20,765 | BMS | | 25,000 | ||||||||
BMS | $ | 99,166 | BMS | $ | 101,620 | Lilly | $ | 19,620 | Amgen | | 17,900 | ||||||||
Celgene | $ | 93,347 | Celgene | $ | 91,033 | BMS | $ | 16,383 | Biogen | | 7,550 | ||||||||
Lilly | $ | 87,343 | Biogen | $ | 84,590 | Biogen | $ | 10,296 | Gilead | | 7,000 | ||||||||
Biogen | $ | 69,916 | Lilly | $ | 78,328 | Celgene | $ | 8,426 | Celgene | | 6,012 | ||||||||
Regeneron | $ | 54,257 | Regeneron | $ | 46,358 | Regeneron | $ | 3,396 | Regeneron | | 3,320 | ||||||||
Alexion | $ | 38,942 | Alexion | $ | 37,041 | Alexion | $ | 2,391 | Alexion | | 2,273 | ||||||||
Vertex | $ | 31,198 | Vertex | $ | 29,201 | United Therapeutics | $ | 1,351 | Vertex | | 1,830 | ||||||||
Incyte | $ | 20,970 | BioMarin | $ | 17,132 | BioMarin | $ | 861 | BioMarin | | 1,681 | ||||||||
BioMarin | $ | 20,820 | Incyte | $ | 15,294 | Alkermes | $ | 648 | Alkermes | | 1,300 | ||||||||
Alkermes | $ | 8,898 | Alkermes | $ | 8,873 | Incyte | $ | 644 | United Therapeutics | | 740 | ||||||||
Alnylam | $ | 8,708 | Alnylam | $ | 8,535 | Vertex | $ | 628 | Incyte | | 588 | ||||||||
United Therapeutics | $ | 6,859 | United Therarapeutics | $ | 7,103 | Alnylam | $ | 62 | Alnylam | | 256 | ||||||||
75th Percentile | $ | 100,201 | | $ | 101,720 | | $ | 19,906 | | | 19,675 | ||||||||
Median | $ | 54,429 | | $ | 57,685 | | $ | 5,409 | | | 4,143 | ||||||||
25th Percentile | $ | 17,839 | | $ | 13,689 | | $ | 647 | | | 1,160 | ||||||||
REGN Percentile Rank | | 50th | | | 48th | | | 47th | | | 48th | ||||||||
| | | | | | | | | | | | | | | | | | | | |
Source: Standard & Poor's Compustat.
*Based on information reported in the companies' most recent Annual Reports on Form 10-K available in September 2015.
The Peer Group utilized in 2015 (approved by the Compensation Committee in September 2015 as noted above) consists of the following 14 companies:
*Regeneron's Biotech R&D Peer.
In making its compensation decisions in December 2015, the Compensation Committee used data from publicly filed proxy statements of the companies in the Peer Group (as compiled by its compensation consultant) to review each element of compensation of our Named Officers against their peers in the Peer Group as well as their total annual compensation in relation to the Peer Group, while taking into account various factors such as the executive's performance, past compensation history, experience, and the role in the Company's success. We use Peer Group data as a point of reference for measurement, but Peer Group data do not represent the only factor considered and there is no targeted pay level percentile. Further, in its review of the Peer Group data, the Compensation Committee also considers the practices of the 10-company sub-group of peers viewed as having businesses and drug discovery cultures that are most similar to Regeneron's, with similarly-sized employee bases (marked with an asterisk
in the table above and referred to as "Biotech R&D Peers"). The Compensation Committee retains discretion in determining the nature and extent of the use of Peer Group data. In addition, in 2015 management and the Compensation Committee reviewed compensation data for biotechnology companies from the 2015 Radford Global Life Sciences Survey (comprising U.S. public biotechnology and pharmaceutical companies that have between 800 and 15,000 employees) to obtain a general understanding of current compensation practices and to assess overall competitiveness of our compensation program.
Compensation Consultant Independence
In accordance with applicable listing standards of the NASDAQ Stock Market LLC and SEC rules, the Compensation Committee evaluated in 2015 the independence of Frederic W. Cook & Co.,
51
Executive Compensation
including by taking into consideration the following factors:
•the fact that Frederic W. Cook & Co. did not provide any other services to the Company;•the amount of fees received from the Company by Frederic W. Cook & Co. as a percentage of its revenue;•the policies and procedures of Frederic W. Cook & Co. designed to prevent conflicts of interest;•the absence of any significant business or personal relationship between Frederic W. Cook & Co. and any member of the Compensation Committee;•the fact that Frederic W. Cook and the representative of Frederic W. Cook & Co. to the Company did not own any stock of the Company; and•the absence of any material business or personal relationship between Frederic W. Cook & Co. and any executive officer of the Company.
The Compensation Committee's evaluation was based in part on a representation letter from Frederic W. Cook & Co.. On the basis of this evaluation, the Compensation Committee concluded that the engagement of Frederic W. Cook & Co. did not raise any conflicts of interest.
To further align the interests of senior management with the interests of shareholders and promote a long-term perspective, the board of directors has adopted stock ownership guidelines for members of senior management, including the Named Officers, and the members of the board of directors. The guidelines are reviewed periodically by the Corporate Governance and Compliance Committee. Pursuant to these guidelines, these individuals are expected to meet share ownership targets that are determined based on their position and their base salary. The share ownership targets are as follows:
•- Chairman of the Board
and Chief Executive Officer, six times (6x) base salary; •Chief Scientific Officer, three times (3x) base salary;•Executive/Senior Vice Presidents, two times (2x) base salary; and•non-employee members ofare also involved in reviewing theboard of directors, six times (6x) the annual retainer.
Covered individuals who do not currently meet these guidelines have five years from becoming subject to the policy to reach their target. Members of senior management who are
hired or promoted, and directors who join the board of directors, have five years from such date to reach their target. Shares held directly, shares held indirectly through our 401(k) Savings Plan, shares held in trust, and shares held by immediate family members residing in the same household are included in determining an individual's share ownership. Unexercised stock options and unvested restricted stock are not considered ownedCompany’s performance for purposes of these guidelines. All directors and officers have either met their respective share ownership targets or are still withinsetting the five-year period for achieving compliance.annual cash incentive.
Say-on-Pay Response
Our shareholders are provided with an opportunity to cast a non-binding, advisory vote every three years on our executive compensation program. Our shareholders most recently had the opportunity to cast advisory say-on-pay votes at our annual shareholder meeting held in June 2014, at which approximately 62% of the votes cast supported the advisory vote proposal on our executive compensation program. Management and the Compensation Committee carefully considered the results of the most recent say-on-pay vote. Senior members of our management as well as the Chairman of the Compensation Committee subsequently spent a significant amount of time speaking with some of our key shareholders about executive compensation and corporate governance. As part of our 2014 engagement effort, we discussed these issues with shareholders collectively representing approximately 47% of the shares of common stock outstanding as of December 31, 2014 (excluding shares held our directors and executive officers and Sanofi). Following the 2014 discussions, we implemented several changes to our executive compensation program and continued the implementation of our existing compensation and governance initiatives. A summary of the relevant changes and initiatives adopted after the most recent say-on-pay vote is provided below.
•We reduced the number of shares underlying the 2014 annual stock option awards to the Named Officers by an average of 16% compared to the prior year (without giving effect to a grant to our Chief Financial Officer, who did not receive an annual stock option award in 2013 because he joined the Company in September 2013).•We eliminated certain perquisites of our Chief Executive Officer and our Chief Scientific Officer we considered no longer consistent with our overall compensation program, including, in the case of our Chief Executive Officer, a tax gross-up related to legal, tax, and financial planning advisory services.
52
Executive Compensation
•We adopted a policy against including excise tax gross-up provisions with respect to payments contingent upon a change in control of Regeneron in contracts, compensatory plans, or other arrangements with the Company's executive officers, including the Named Officers (other than the existing employment agreement with our Chief Executive Officer or any amendments thereto, which we expressly exempted).
•We provided additional information regarding compensation decisions and our compensation philosophy in the Compensation Discussion and Analysis of our 2015 proxy statement to better communicate to our shareholders what drives compensation decisions at Regeneron.
We will continue to consider the outcome of our past and future advisory vote results. Our shareholder engagement efforts and implementation of compensation and governance initiatives continued in 2015, as described further below under "2015 Shareholder Outreach."
2015 Shareholder Outreach
We have instituted an ongoing shareholder outreach program through which we seek input frommake our institutional investors and other shareholders regarding our executive compensation and other governance practices, and implement appropriate changes based on this input. We value shareholder views and insights and believe that constructive and meaningful dialogue allows us to develop broader relationships with investors over the long-term and builds informed relationships that promote transparency and accountability. We continued our shareholder outreach efforts in 2015 and engaged in discussions with shareholders collectively representing approximately 47% of the shares of common stock outstanding as of December 31, 2015 (excluding shares held our directors and executive officers and Sanofi). Below is a summary of recent changes we have adopted based on shareholder feedback and other relevant considerations:
Consideration of Risk in Company Compensation Policies
The Compensation Committee regularly reviews the Company's compensation and benefits programs, including its executive compensation program and its incentive based compensation programs for commercial personnel. Our compensation and governance-related policies are further enhanced by our stock ownership guidelines applicable to our senior officers and our policy regarding recoupment or reduction of incentive compensation of our officers and other specified employees for compliance violations, as well as a policy against hedging and pledging of our securities by our directors and employees, including the Named Officers. We have also adopted a policy against including excise tax gross-up provisions with respect to payments contingent upon a change in control of Regeneron in
contracts, compensatory plans, or other arrangements with the Company's executive officers, including the Named Officers (other than the existing employment agreement with our Chief Executive Officer or any amendments thereto, which we expressly exempted). These policies evidence Regeneron's continued commitment to robust corporate governance and are meant to reduce compensation-related risks and ensure greater alignment of the interests of our employees, including the Named Officers, and those of the Company and our shareholders.
We believe that the Company's programs balance risk and potential reward in a manner that is appropriate to the Company's circumstances and in the best interests of the Company's shareholders over the long term. We also believe that
53
Executive Compensation
the Company's compensation and benefits programs do not create risks that are reasonably likely to have a material adverse effect on the Company.
Tax Implications
Section 162(m) of the Internal Revenue Code limits the deductibility for federal income tax purposes of compensation in any year paid to the Chief Executive Officer and the other Named Officers (other than the Chief Financial Officer) to the extent such compensation exceeds $1 million and does not qualify as "performance-based" compensation as defined under Section 162(m) of the Internal Revenue Code. The Company has adopted (and the shareholders approved at the 2015 annual shareholder meeting) the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan. Awards under the Plan (as well as awards under the previously approved Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan) may, but
are not required to, be subject to the attainment of performance goals in order to qualify for this performance-based compensation exception. The Compensation Committee has implemented the Cash Incentive Bonus Plan for annual cash bonuses of the Named Officers in respect of performance in 2016.
The Compensation Committee takes into account the deductibility of compensation in determining Named Officer compensation. However, the Compensation Committee reserves the right to use its judgment to authorize compensation payments that are not deductible, such as when the Compensation Committee believes that such payments are necessary to maintain the flexibility needed to attract talent, promote executive retention, or reward performance, or as required to comply with the Company's contractual commitments. As noted above, compensation attributable to stock options generally qualifies as "performance-based" compensation and, as such, receives favorable treatment under Section 162(m) of the Internal Revenue Code.
|
Our executive compensation program currently has five components:
•base salary;•annual cash bonus;•- annual equity awards
typically consisting of stock options; •certain perquisites and other personal benefits; and•potential severance benefits.
Base Salary
We provide the Named Officers and other employees with base salary to compensate them for services rendered during the fiscal year and provide them withon a regular, monthly income. In determining base salaries for our Named Officers, the Compensation Committee considers the executive's scopepre-set schedule. The meetings at which such grants are approved are generally scheduled well in advance of responsibilities, experience, annual performance, and future potential or role in future success. The Compensation Committee also considers base salaries for comparable positions in our geographic region, competitive salary practices of companies in the Peer Group and the broader biopharmaceutical industry, and annual inflation levels.
For each of 2015 and 2016, each of the Named Officers received a merit increase of 3.5% of his 2014 and 2015 base salary, respectively (which were in addition to the other increases discussed for the Named Officers below). In addition, for 2015, Dr. Schleifer received a base salary increase of
$91,300, which aligned his base salary with that of the median of the companies included in the 2014 Radford Global Life Sciences Survey and below the median of the Peer Group (as in effect in 2014), and Dr. Yancopoulos received a base salary increase of $77,600 to set his base salary at 85% of Dr. Schleifer's. Dr. Yancopoulos's cash compensation, including base salary, is set at 85% of Dr. Schleifer's, which we believe is more appropriate for him in light of the absence of meaningful comparative data for similarly situated executives. Further, for 2015, Dr. Stahl received a $39,200 base salary increase in connection with his promotion to Executive Vice President, Research and Development and Mr. Terifay received a $50,000 base salary increase, in each case to better align his base salary with relevant industry comparative information. For 2016, Dr. Stahl and Mr. Landry received a $50,000 base salary increase each in order to better align their base salaries with relevant industry comparative information. The determinations regarding the 2015 and 2016 merit increases were based on broad-based national data for high-performing, larger biotechnology companies. In each of 2015 and 2016, the base salaries of the Named Officers were set at or below the median of the Peer Group (other than with respect to Dr. Yancopoulos, whose cash compensation, including base salary, is set at 85% of Dr. Schleifer's (as noted above)).
54
Executive Compensation
Annual Cash Bonus
It has been our practice to offer annual cash bonus opportunities to our Named Officers. The Compensation Committee focuses exclusively on our overall corporate performance when determining the annual cash bonus for our Chief Executive Officer and Chief Scientific Officer. The cash bonuses of the other Named Officers are based both on overall corporate performance and their individual contributions and performances during the year. We historically had no formal bonus plan and the award of any bonus to a Named Officer was based on an assessment of corporate and, in the case of Named Officers other than Dr. Schleifer, individual performance. We believe that this approach, rather than working from a rigid bonus formula or plan, was beneficial given the growth trajectory of the Company and its rapid transformation over the past few years from a development-stage company to a fully integrated, commercial-stage biopharmaceutical company. In 2015, the Company adopted, and the shareholders approved, the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan. The Compensation Committee has implemented the Cash Incentive Bonus Plan for annual cash bonuses of the Named Officers in respect of performance in 2016.
The 2015 cash bonus target for the Chief Executive Officer was 100% of his base salary. In December 2015, the board of directors approved an increase in the Chief Executive Officer's cash bonus target to 120% of his base salary to bring Dr. Schleifer's target annual cash compensation to the median of the companies included in the 2015 Radford Global Life Sciences Survey. For 2016, Dr. Schleifer's target cash compensation remained below the median of the Peer Group. Consistent with Regeneron's historical practice, the increase was given effect in the calculation of Dr. Schleifer's cash bonus paid in respect of 2015. The Chief Executive Officer
recommended 2015 target bonuses for the other Named Officers, which were reviewed and approved by the Compensation Committee. In December 2015, the Compensation Committee increased the cash bonus target for Mr. Landry and Mr. Terifay to 50% and 60% of their respective base salaries; consistent with Regeneron's historical practice, these increases were given effect in the calculation of their cash bonuses paid in respect of 2015. In determining the cash bonus targets for 2015 for Mr. Landry, Dr. Stahl, and Mr. Terifay, the Compensation Committee took into consideration the compensation of similarly situated executive officers at companies in the Peer Group and, in the case of Mr. Terifay, also his promotion to Executive Vice President, Commercial.
In determining the cash bonus target for Dr. Yancopoulos, the Compensation Committee took into consideration the importance of his scientific leadership as President of Regeneron Laboratories and Chief Scientific Officer and the significant contributions he has made to the success of the Company and, specifically, to the discovery and development of the Company's commercial products, its pipeline of internally developed product candidates, and its platform technologies.
The Compensation Committee determined that for Dr. Yancopoulos there were no meaningful comparative data relating to similarly situated executives and that his base salary, cash bonus, and annual stock option awards would be set at 85% of Dr. Schleifer's. The 2015 cash bonus target for Dr. Yancopoulos was 120% of his base salary (increased in connection with the increase of Dr. Schleifer's cash bonus target as described above). These cash bonus targets are consistent with the Company's emphasis on performance-based compensation and are set at or below the median of the Peer Group (other than with respect to Dr. Yancopoulos, whose cash compensation, including cash bonus, is set at 85% of Dr. Schleifer's).
In December 2015, our Named Officers were awarded the following cash bonuses, which were paid in January 2016.
Named Officer | | Bonus Target (as percentage of base salary) | | Personal Performance Multiplier | | Company Performance Multiplier | | Total Cash Bonus ($) | ||||||
Leonard S. Schleifer, M.D., Ph.D. | | 120% | | n/a | | 2.0 | | 2,880,000 | | |||||
George D. Yancopoulos, M.D., Ph.D. | | 120% | | n/a | | 2.0 | | 2,448,000 | | |||||
Robert E. Landry | | 50% | | 1.5 | | 2.0 | | 465,750 | 1 | | ||||
Neil Stahl, Ph.D. | | 60% | | 1.5 | | 2.0 | | 594,000 | 1 | | ||||
Robert J. Terifay | | 60% | | 1.5 | | 2.0 | | 574,668 | 1 | | ||||
| | | | | | | | | | | | | | | |
1Amount based on Named Officer's cash bonus target and his weighted average performance with a weight of 40% for personal performance and 60% for Company performance.
The cash bonuses were determined through the use of both an individual and a Company performance component with a possible
range of 0 to 1.5 for the personal performance multiplier and a possible range of 0 to 2.0 for the Company performance multiplier,
55
Executive Compensation
depending upon performance during the year. Both the personal performance multiplier and the Company performance multiplier were determined by the Compensation Committee for each Named Officer based on the Committee's assessment of the Company's performance relative to the general corporate goals described below and, in the case of each of Mr. Landry, Dr. Stahl, and Mr. Terifay, the Named Officer's personal performance during the year.
The Compensation Committee determined that the Company's performance in 2015 well exceeded its 2015 corporate goals. These corporate achievements included the following:
•47% growth in EYLEA® global net product sales as compared to 2014;•46% growth in our total revenues as compared to 2014;•19% growth in non-GAAP net income as compared to 2014 (non-GAAP net income is not a measure calculated in accordance with U.S. Generally Accepted Accounting Principles; see Appendix A for a definition of non-GAAP net income and a reconciliation of non-GAAP net income to net income);•advances in our EYLEA® franchise, including regulatory approval of EYLEA® for the treatment of visual impairment due to macular edema secondary to retinal vein occlusion and the treatment of visual impairment secondary to myopic choroidal neovascularization in the European Union; regulatory approval of EYLEA® for the treatment of diabetic retinopathy in patients with diabetic macular edema in the United States; and regulatory approval of EYLEA® for the treatment of retinal vein occlusion in Japan;•approval and launch of Praluent® (alirocumab) Injection, the first FDA-approved drug in a new class of drugs that lower LDL ("bad") cholesterol;•positive Phase 3 data for sarilumab from three Phase 3 studies in patients with rheumatoid arthritis (SARIL-RA-TARGET, SARIL-RA-EASY, and SARIL-RA-ASCERTAIN) and submission of a Biologics License Application for sarilumab with the FDA;•positive pivotal Phase 2b data for dupilumab in asthma and completion of enrollment of the dupilumab atopic dermatitis Phase 3 studies;•new collaboration agreement relating to fasinumab with Mitsubishi Tanabe Pharma Corporation for Japan, Korea, and nine other Asian countries, excluding China;•initiation of Phase 3 clinical study of REGN2222 for Respiratory Syncytial Virus;•continued growth of our clinical development pipeline, as evidenced by the submission of one Investigational New Drug Application with the FDA in 2015 and 13 product
candidates (consisting of one Trap-based and 12 fully-human monoclonal antibody product candidates based on the Company'sVelocImmune® technology) in clinical development as of December 31, 2015;
•new global strategic collaboration with Sanofi to discover, develop, and commercialize antibody-based cancer treatments in the field of immuno-oncology; and•further important steps to support our current and future growth, including adding two new buildings in the Tarrytown campus providing nearly 300,000 square feet of additional laboratory and office space; significant progress with the construction of a new manufacturing facility in Limerick, Ireland; and increasing headcount on a year-over-year basis by approximately 47% as of December 31, 2015.
See "Section 1 – Summary – 2015 Performance Overview" above for additional information.
In recognition of these significant achievements, the Company performance multiplier for 2015 was set at 2.0.
With respect to 2015, the Compensation Committee approved a personal performance multiplier of 1.5 for each of Mr. Landry, Dr. Stahl, and Mr. Terifay. The personal performance component accounted for 40% of these officers' bonuses. The Company component was based on a Company performance multiplier that was determined based on the Company's overall corporate performance (as described above) against 2015 goals that were approved by the board of directors in January 2015. This Company performance component accounted for 60% of the bonuses awarded to Mr. Landry, Dr. Stahl, and Mr. Terifay. In the case of Drs. Schleifer and Yancopoulos, the Compensation Committee focused exclusively on our overall Company performance in 2015 (as described above) when determining their cash bonuses and did not utilize a personal performance multiplier.
In determining the personal performance multiplier for Mr. Landry, the Compensation Committee gave special consideration to Mr. Landry's leadership of and accomplishments in the Company's accounting and finance functions and his assumption of additional responsibilities since he joined the Company in September 2013. In the case of Dr. Stahl, the Compensation Committee focused on the progress and continued expansion of the Company's preclinical and clinical development pipeline, including the positive results from the Company's clinical trials reported in 2015, as summarized in "Section 1 – Summary – 2015 Performance Overview" above. In the case of Mr. Terifay, the Compensation Committee focused primarily on the continued commercial success of EYLEA® and the fact that EYLEA® U.S. net product sales in 2015 grew by 54% compared to 2014; the launch of Praluent® in 2015; and his leadership of the Company's commercial group, including the increase in his responsibilities in
56
Executive Compensation
connection with the expansion of the commercialization group to support the launch of Praluent® and the planned launches of sarilumab and dupilumab.
Annual Stock Option Awards
We have used stock option grants as the primary vehicle for offering long-term incentives and rewarding our Named Officers and other eligible employees. These time-based stock options generally vest at a rate of 25% per year over the first four years of the ten-year option term, subject to the executive's continued employment. We grant stock option awards to our Named Officers and other eligible employees based on their annual performance and their position and responsibilities with the Company. Each of our Named Officers generally receives an annual stock option grant and all of our other regular employees are also eligible for an annual stock option grant, subject to satisfactory performance. The number of stock options granted to each Named Officer is determined on a discretionary basis, rather than by a formula. The Compensation Committee primarily considers the number of shares underlying the awards relative to the number of basic shares of common stock outstanding and not the grant date, fair value of the award (as determined accordingwithout regard to the Black-Scholes model). The Compensation Committee is therefore able to evaluate such grants on a consistent basis as compared to other companies and regardlesstiming of fluctuations in the price of Regeneron'searnings or other companies' common stock. Further, focusing on the number of shares and the incremental sharing rate of potential future upside (rather than targeting a specific Black-Scholes grant date fair value) avoids rewarding officers with larger grant sizes following a decline in our stock price. While the Compensation Committee takes the estimated Black-Scholes grant date fair value of annual stock option grants into account, it does not necessarily determine its compensation decisions.
It has been the practice of our Compensation Committee tomajor announcements. We generally grant annual stock optionequity awards to eligible employees whose performance is determined to merit an annual grant, including the Named Officers,NEOs, at a meeting held during December. In 2015, stock option awards (all of which were non-qualified stock options) were granted to our Named Officers and other eligible employees on December 16, 2015.
Pursuant to the terms of the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, stock option awards are granted for so long as our common stock is listed on the NASDAQ Global Select Market, with an exercise price equal to the
average of the high and low sales price per share of our common stock as quoted on the NASDAQ Global Select Market on the date of the grant or, if such date is not a trading day, on the last preceding date on which there was a sale of our common stock on the NASDAQ Global Select Market.
All
We periodically evaluate the personal benefits and perquisites afforded to our NEOs. The Compensation Committee also regularly meets in executive session to discuss any of the stock options granted tomatters that fall within its responsibilities.
Members of our Named Officerssenior management play a role in 2015 will vest ratably over a periodthe overall executive compensation process and assess performance of four years. Except as set forth below underother officers. They also recommend for the heading "Post-Employment Compensation" on page 66, stock option vesting ceases,Compensation Committee’s approval the salary, cash incentive, and unvested stock options are forfeited, upon termination of employment. The use of only time-based stock options is consistent with the Company's practice prior to 2008equity grant budgets for non-officers and since 2012.
Our Named Officers received a grant of time-based stock options on December 16, 2015, as set forth below. The annualmake specific recommendations for salary increases, cash incentives, and equity grants shown below reflected an average reduction of 15% compared to 2014for other officers. For our NEOs (other than Mr. Terifay's award, which remained at the 2014 level dueour CEO), recommendations to his promotion to Executive Vice President, Commercial). This decrease constituted the third consecutive double-digit percentage decrease in the annual grant of stock options to our Named Officers, in each case following outstanding TSR performance. In reducing the size of 2015 annual stock option awards to the Named Officers, the Compensation Committee soughtregarding their compensation are made by, or with the approval of, our CEO, who also evaluates their performance. Our CEO’s performance is evaluated directly by the Compensation Committee based on the Company’s overall corporate performance against annual goals that are approved by the board of directors at the beginning of each year, as discussed above.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 77 / 77 |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
SHAREHOLDER INPUT AND OUTREACH
We believe in casting a broad net for information, and think it is very important to reducereach out to our shareholders for ideas, input, and direct feedback. We do this formally through our triennial say-on-pay votes, as well as through discussions with our shareholders both in connection with our annual shareholder meetings and in the potential dilutive impact“off-season,” where we discuss compensation, governance, and other issues of new equity awards without adversely affectingimportance and interest to our shareholders. In addition, on a more informal basis, we engage with our shareholders through regular investor relations channels, governance engagements, industry conferences, and informal exchanges in other settings.
Say-on-pay vote.Our shareholders are provided with an opportunity to cast a non-binding, advisory vote every three years on our executive compensation program. You will have that opportunity again at the effectiveness2020 annual shareholder meeting. Our most recent advisory say-on-pay vote was held at our 2017 annual shareholder meeting, at which this advisory proposal was approved by 67% of the votes cast. As has been the case with previous say-on-pay votes, management and the Compensation Committee carefully considered the result of this say-on-pay vote and subsequently solicited further feedback from our key shareholders on compensation and governance matters. In the last several years, following our two most recent advisory say-on-pay votes, we implemented several changes to our executive compensation program and continued the implementation of our existing compensation and governance initiatives. These and other recent changes are summarized in the table below following the description of our shareholder outreach.
Shareholder outreach.In addition to the more formal input of the say-on-pay vote discussed above, we maintain a robust shareholder outreach program (both in connection with our annual shareholder meetings and in the “off-season”) through which has successfully motivatedwe seek input from shareholders and environmental, social, and governance (“ESG”)-focused groups regarding our seniorexecutive compensation and other governance practices, and implement appropriate changes based on this input.
Our annual shareholder outreach program is quite extensive – last year we approached shareholders collectively representing nearly 60% of the shares of common stock outstanding as of December 31, 2019 (excluding shares held by our directors and executive officers and Sanofi), which we refer to as “public shareholders”. As part of our outreach, we engaged in direct one-on-one discussions with over 50% of our public shareholders as well as both leading proxy advisory firms. We encourage director participation in our outreach, and our Compensation Committee Chair led many of these discussions. Shareholder feedback is discussed with management teamand, depending on the topic, relayed for consideration to deliver high operating performancethe appropriate committee of the board of directors (typically the Compensation Committee or the Corporate Governance and Compliance Committee), the full board, or both. In recent years, shareholder value. The reduction also tookinput resulted in specific changes to our compensation and corporate governance practices and policies, including the changes discussed below.
Our 2019 outreach discussions built on an active outreach program in prior years and focused on, among other matters, equity compensation matters, including investor views on performance-based equity, the appropriate scope of equity compensation programs, and acceptable burn rate and dilution. As discussed elsewhere in this CD&A, influenced by shareholder feedback and input from the past several years, in December 2019 we introduced PSUs as a component of the annual equity awards for our CEO and CSO (as well as the Chairman of the Board). This change and other changes we have adopted in recent years while taking into account the increaseshareholder feedback, which we believe demonstrate our continued commitment to good governance, are outlined in the Company's stock price in recent years. We will continue to assess the number of stock options that will be awarded to the Named Officers as annual merit grants.table below.
78 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
Recent Changes Adopted Based on Shareholder Feedback
| What We Heard | What We Did | When We Did It | ||
| Concern about size of NEO equity awards | Implemented seven consecutive reductions in the number of option-equivalent shares underlying the annual equity awards to most of our NEOs, regardless of the stock price on the grant date | 2013–2019 | ||
| Support for all-employee equity compensation strategy, but concern about resulting burn rate | Maintained our all-employee equity compensation strategy while addressing concerns about the resulting burn rate using a two-part approach:
2 Introduce (starting in 2019) full-value awards for a portion of the annual equity awards to eligible employees: PSUs for 30% of CEO/CSO/Chairman 2019 annual equity awards; RSAs/RSUs for 30% of 2019 annual equity awards for other NEOs and certain other executives; and RSAs/RSUs for 40% of 2019 annual equity awards for other employees. | 2013–2019 | ||
These changes have allowed us to maintain relatively stable burn rate despite a 316% increase in the number of employees from the end of 2012 through 2019. 2019 burn rate was lower by 1.1% despite hiring 730 new employees during the year. | ||||
| Preference for further pay-for-performance alignment | As part of the 2019 annual equity awards to our CEO and CSO, we introduced PSUs with rigorous performance goals tied to the Company’s TSR (target = 61% TSR over five years). | 2019 | ||
| Desire for more clarity regarding the process for determining annual cash incentives | Enhanced the existing process for determining 2019 annual cash incentives and provided more detailed disclosure about the Compensation Committee’s determination of the Company performance multiplier. See “Components of Executive Pay: What We Pay and Why We Pay It – Annual Cash Incentives” above for more information. | 2019–2020 | ||
| Concern about compensation program for non-employee directors and the Chairman of the Board | Introduced a new compensation program for our non-employeedirectors and the Chairman of the Board in November 2018. Asa result:
• Introduced full-value awards (RSUs) as part of thenon-employee director equity awards. | 2018 |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 79 / 79 |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
| Requests for additional information with respect to ESGmatters | Conducted ESG audit in 2017 to identify potential gaps withrespect to ESG matters. Increased the breadth and depth of ESG data collectionand reporting, including introducing first comprehensive Responsibility Report in 2018, and actively engaged withvarious ESG ratings agencies in 2018, 2019, and 2020 to date,resulting in significant improvements to several ESG scores. Built an ESG strategy operational structure, including byinstituting board-level oversight and establishing a cross-functional responsibility committee comprised of managementmembers. Announced our support for the United Nation’s SustainableDevelopment Goals, disclosing where we plan to focus todeliver the most impact. Set bold, new ESG goals, spanning across the environmentaland social issues that are most significant to our business andstakeholders. | 2017–2020 |
| Concern about executiveperquisites | Eliminated certain CEO non-business perquisites, includinga tax gross-up related to legal, tax, and financial planningadvisory services | 2014 |
| Payments contingent on changein control should not benefitfrom excise tax gross-ups | Adopted a new policy against including excise tax gross-upprovisions with respect to payments contingent upon achange in control of Regeneron in contracts, compensatoryplans, or other arrangements with the Company’s executiveofficers, including the NEOs (grandfathered the CEOemployment agreement, which has not been amended since) | 2014 |
| The Company should beable to claw back incentivecompensation in case ofemployee misconduct | Adopted a recoupment (clawback) policy covering bothfinancial and non-financial misconduct | 2014 |
| Need to communicate moreclearly about compensationmatters and link to Regeneron’sbusiness model andperformance | Substantially revised CD&A section of proxy statement andprovided additional information regarding compensationdecisions and our compensation philosophy | 2014–2020 |
We also provide all shareholders and others a means to contact us at any time. That information is included in this proxy statement—see “Shareholders—Shareholder Communications.” We welcome your input.
80 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| COMPENSATION-RELATED MATTERS / COMPENSATION DISCUSSION AND ANALYSIS |
INDEPENDENT COMPENSATION CONSULTANT
The Compensation Committee has the sole authority to retain, at the Company’s expense, one or more third-party compensation consultants to assist the Compensation Committee in performing its responsibilities and to terminate the services of the consultant if the Compensation Committee deems it appropriate. In 2019, the Compensation Committee (and, as discussed above with respect to non-employee director compensation matters, the Corporate Governance and Compliance Committee) utilized the services of Frederic W. Cook & Co. In order to maintain its independence, the Compensation Committee retained Frederic W. Cook & Co. directly and Frederic W. Cook & Co. performed services for the Compensation Committee exclusively at the Compensation Committee’s direction. The Compensation Committee periodically evaluates the independence of its compensation consultant. In accordance with applicable listing standards of the NASDAQ Stock Market LLC and SEC rules, in 2019 the Compensation Committee evaluated the independence of Frederic W. Cook & Co., including by taking into consideration the following factors:
| • | the fact that Frederic W. Cook & Co. did not provide any other services to the Company (other than those provided to the Corporate Governance and Compliance Committee); | |||
| the amount of fees received from the Company by Frederic W. Cook & Co. as a percentage of its revenue; | |||
| the policies and procedures of Frederic W. Cook & Co. designed to prevent conflicts of interest; | |||
| the absence of any significant business or personal relationship between Frederic W. Cook & Co. representatives and any member of the Compensation Committee; | |||
| the fact that Frederic W. Cook and the representatives of Frederic W. Cook & Co. to the Company did not own any stock of the Company; and | |||
1These stock options all have an exercise priceThe Compensation Committee’s evaluation was based in part on a representation letter from Frederic W. Cook & Co. On the basis of
$555.67 per share,this evaluation, theaverageCompensation Committee concluded that the engagement of Frederic W. Cook & Co. did not raise any conflicts of interest.The Compensation Committee’s consultant reviews management recommendations for compensation plans, budgets, and strategies, and also advises the Compensation Committee on how regulations and trends in executive compensation nationally and specifically in the pharmaceutical and biopharmaceutical industries may be relevant to the Company. It also assists with developing the Peer Group, provides comparative compensation information for our CEO and CSO and the other members of the
highBoard (using the Peer Group andlow sales price per shareother compensation data as described below); reviews senior management’s compensation recommendations for other officers, including the other NEOs; and provides general advice to the Compensation Committee on compensation matters, including facilitating the articulation and periodic review of the Company’s compensation philosophy or replenishment of ourcommon stocklong-term equity incentive plan.For purposes of setting our NEOs’ and other senior executives’ compensation, we use comparative compensation information from a relevant peer group of companies (referred to in this proxy statement as
quoted“Peer Group”). We select the companies in the Peer Group with the assistance of Frederic W. Cook & Co. based on factors including, but not limited to, theNASDAQ Global Select Market on the date of grant.
57following:
Executive Compensation
| • | research and development orientation; | • | stage of development; and | |||
| market capitalization; | ||||||
|
| |||||
| • | number of employees; |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 81 / 81 |
COMPENSATION-RELATED MATTERS/COMPENSATION DISCUSSION AND ANALYSIS
Perquisites and Other Personal Benefits
The Named Officers are providedPeer Group is also meant to provide a representative sample of companies with a limited numberwhich we compete for talent. We periodically reassess the composition of perquisitesthe Peer Group and other personal benefits. make changes as appropriate, taking into account factors such as changes in the Company’s market capitalization and merger-and-acquisition activity impacting the existing Peer Group companies.
The Peer Group utilized in 2019 consists of the following 14 companies:
| AbbVie Inc. | Biogen Inc.* | Gilead Sciences, Inc.* | ||
| Alexion Pharmaceuticals, Inc.* | BioMarin Pharmaceutical Inc.* | Incyte Corporation* | ||
| Alkermes plc* | Bristol-Myers Squibb Company | United Therapeutics Corporation* | ||
| Alnylam Pharmaceuticals, Inc.* | Celgene Corporation *+ | Vertex Pharmaceuticals, Inc.* | ||
| Amgen Inc. | Eli Lilly and Company |
| * | Regeneron’s Biotech R&D Peer. |
| + | Acquisition by Bristol-Myers Squibb Company was completed in November 2019. |
The Compensation Committee periodically reviewsreviewed the perquisitesPeer Group in June 2019 and, other personal benefitsbased on the recommendation of Frederic W. Cook & Co., did not make any changes. As part of its assessment, the Compensation Committee took into account that Regeneron was the median company (or just below the median) in the Peer Group based on market capitalization, revenues for the last four completed quarters, and the then-available reported number of employees, based on the data provided to the Named Officers,Committee, as shown in the table below.
| Market Capitalization ($ Millions) | Revenues ($ Millions) | Employees | ||||||||
| As of 5/31/19 | Trailing 12-Month Average | Last Four Quarters | (as of last 10-K filing) | |||||||
| AbbVie | $113,403 | AbbVie | $150,796 | AbbVie | $32,647 | Lilly | 38,680 | |||
| Lilly | $106,761 | Amgen | $126,120 | Lilly | $24,684 | AbbVie | 30,000 | |||
| Amgen | $101,676 | Gilead | $95,995 | Amgen | $23,750 | Bristol-Myers Squibb | 23,300 | |||
| Gilead | $79,154 | Lilly | $95,330 | Bristol-Myers Squibb | $23,288 | Amgen | 21,500 | |||
| Bristol-Myers Squibb | $74,213 | Bristol-Myers Squibb | $94,396 | Gilead | $22,320 | Gilead | 11,000 | |||
| Celgene | $66,146 | Biogen | $64,836 | Celgene | $15,768 | Celgene | 8,852 | |||
| Vertex | $42,562 | Celgene | $62,166 | Biogen | $13,812 | Biogen | 7,800 | |||
| Biogen | $42,519 | Vertex | $42,658 | Regeneron | $6,911 | Regeneron | 7,448 | |||
| Regeneron | $32,938 | Regeneron | $37,884 | Alexion | $4,341 | BioMarin | 2,849 | |||
| Alexion | $25,491 | Alexion | $26,721 | Vertex | $3,265 | Alexion | 2,656 | |||
| lncyte | $16,861 | BioMarin | $16,298 | lncyte | $1,997 | Vertex | 2,500 | |||
| BioMarin | $14,727 | lncyte | $15,452 | United Therapeutics | $1,601 | Alkermes | 2,300 | |||
| Alnylam | $7,193 | Alnylam | $10,145 | BioMarin | $1,519 | lncyte | 1,367 | |||
| United Therapeutics | $3,679 | Alkermes | $7,138 | Alkermes | $1,092 | Alnylam | 1,065 | |||
| Alkermes | $3,380 | United Therapeutics | $5,173 | Alnylam | $86 | United Therapeutics | 860 | |||
| 75th Percentile | $84,785 | $95,499 | $23,404 | 21,950 | ||||||
| Median | $42,541 | $52,412 | $9,076 | 5,325 | ||||||
| 25th Percentile | $12,843 | $14,125 | $1,581 | 2,067 | ||||||
| Regeneron Percentile Rank in Peer Group | 43P | 45P | 48P | 53P | ||||||
Source: Standard & Poor’s Capital IQ.
82 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DISCUSSION AND ANALYSIS
Further, in our review of the Peer Group data, we also consider the practices of the 10-company sub-group of peers viewed as having businesses and drug discovery and development cultures that are most similar to Regeneron’s, with similarly-sized employee bases (marked with an asterisk in the table above and referred to as “Biotech R&D Peers”).
In making the compensation decisions in December 2019, we used data from publicly filed proxy statements of the companies in the Peer Group (as compiled by the Compensation Committee’s compensation consultant) to review each component of compensation of our NEOs against their peers in the Peer Group as well as their total annual compensation in relation to the Peer Group, while taking into account various factors such as the executive’s performance, past compensation history, experience, and their role in the Company’s success. We use Peer Group data as a point of reference for measurement, but Peer Group data do not represent the only factor considered and there is no targeted pay level percentile. The Compensation Committee retains discretion in determining the nature and extent of the use of Peer Group data.
We believe that the Company’s programs balance risk and potential reward in a manner that is appropriate to the Company’s circumstances and in the best interests of the Company’s shareholders over the long term. We also believe that the Company’s compensation and benefits programs do not create risks that are reasonably likely to have a material adverse effect on the Company. We regularly review the Company’s compensation and benefits programs, including its executive compensation program and its incentive-based compensation programs (such as sales incentive plans). The Company’s compensation and governance-related policies are further enhanced by our stock ownership guidelines applicable to our senior officers and our policy regarding recoupment or reduction (clawback) of incentive compensation of our officers and other specified employees for compliance violations; as well as a policy against hedging and pledging of our securities by our directors and employees, including the Chief Executive Officer.
AllNEOs. We also have a policy against including excise tax gross-up provisions with respect to payments contingent upon a change in control of Regeneron in contracts, compensatory plans, or other arrangements with the Named Officers are eligibleCompany’s executive officers, including the NEOs (other than the existing employment agreement with our CEO or any amendments thereto, which we expressly exempted). These policies demonstrate Regeneron’s continued commitment to receive financial and tax planning assistance, which are taxable benefitsrobust corporate governance and are meant to savereduce compensation-related risks and ensure greater alignment of the executives timeinterests of our employees, including the NEOs, and those of the Company and our shareholders.
We take tax considerations into account in making our compensation-related assessments and decisions.
Prior to the enactment of the Tax Cuts and Jobs Act in December 2017, Section 162(m) of the Internal Revenue Code generally limited the deductibility for federal income tax purposes of compensation in any year paid to the CEO and the other NEOs (other than the Chief Financial Officer) (the “covered employees”) to the extent such compensation exceeded $1 million, subject to certain exceptions. “Performance-based” compensation, as defined under Section 162(m) of the Internal Revenue Code, was exempt from such deduction limitation if specified requirements set forth in the Internal Revenue Code and applicable Treasury regulations were met. The Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan and the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, each adopted prior to the enactment of the Tax Cuts and Jobs Act, allow (but do not require) awards thereunder to be subject to the attainment of performance goals in order to focus on business mattersqualify for this performance-based compensation exception.
Under the Tax Cuts and Jobs Act, which became effective for us commencing with our 2018 fiscal year, the exception under Section 162(m) for performance-based compensation is no longer generally available, subject to transition relief for certain grandfathered arrangements in effect as well asof November 2, 2017. Further, the definition of covered employees has been expanded to recognize that their tax situations are affected by their employment at Regeneron. Similar to other employees, the Named Officers may participate in company-wide health, disability, life insurance, and other benefit plans, as well asinclude our 401(k) Savings Plan. All employees who participate in our 401(k) Savings Plan are eligible to receive certain matching contributions.CFO. In each plan year, we contribute to each participant's account a matching contribution (in the form of sharesaddition, once one of our common stock) equalNEOs is considered a covered employee subject to 50%the deduction limitation of Section 162(m), the NEO will remain a specified percentagecovered employee so long as he or she receives compensation from us. Despite the elimination of the participant'sperformance-based compensation thatexception, we have continued to use the participant has contributedCash Incentive Bonus Plan for annual cash incentives of the NEOs because we believe it furthers our compensation philosophy and objectives regardless of tax treatment. If shareholders approve the proposed Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan at the 2020 annual
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 83 / 83 |
COMPENSATION-RELATED MATTERS/COMPENSATION DISCUSSION AND ANALYSIS
shareholder meeting, the Plan’s provisions relating to the plan (which was 6% with respectperformance-based compensation exception under Section 162(m) will be eliminated. The Compensation Committee will continue to eachreview the full impact of 2013Section 162(m) (as revised by the Tax Cuts and 2014Jobs Act) on the Company, our compensation programs, and 8% with respectexecutive compensation trends generally as it makes compensation-related assessments and decisions in the future.
Due to
2015), up to a maximum level established under the requirements set forth in Section 274(e)(2) of the Internal Revenue Code. In addition, for 2014,Code, Company-provided personal and guest air travel (which is provided by the Company made an additional discretionary contribution equal to 1% of each eligible employee's salary. Each of our Named Officers participated in our 401(k) Savings Plan during 2015 and received a matching contribution in the aggregate amount of $10,600 in the form of shares of our common stock. The contribution was paid in February 2016 and is included in the compensation amounts reported for each of our Named Officers in the Summary Compensation Table included in this proxy statement. As with all employees, the number of shares of common stock that each Named Officer received was determined using the average market price per share of our common stock during the 401(k) Savings Plan year, which for 2015 was $498.52.
Our Chief Executive Officer is entitled to life insurance, long-term disability, and medical malpractice insurance premiums (as well as an additional amount for tax preparation and financial planning services) pursuant to the terms of his employment agreement, as described in footnote 5 to the Summary Compensation Table included in this proxy statement. Pursuant to the terms of our security policy, the Company's Chief Executive Officer and Chief Scientific Officer are
58
Executive Compensation
required,only to the extent practicable, to utilize personal travelpermitted under board-approved guidelines and a security services, on-site residential security at their primary residence, and secure car transportation. We calculatepolicy adopted by the aggregate incremental cost to Regeneron for secure car transportation as the portion of the invoiced amount from our third-party provider of such transportation that is attributable to personal useboard based on the number of hours used, without including fixed costs that would be incurred in any event.
In addition, in order to ensure increased efficiencies and to provide a more secure traveling environment, the Company utilizes on-demand air transportation for certain executive and director travel in accordance with guidelines approved by our board of directors. Based on the recommendation of an independent, third-party security study,study) results in a partial disallowance of the guidelines and our security policy (as amendedrelated corporate tax deductions. In 2019, this disallowance amounted to approximately $1.9 million.
We take into account the deductibility of compensation in November 2015) require Drs. Schleifer and Yancopoulos (as well as their spouses and dependent children when they accompany them)determining NEOs’ compensation. However, we reserve the right to use as much as practicable, Company-provided aircraft for all business and personal air travel. Starting in 2016, Regeneron will cover the cost of any such personal air travel for upour judgment to $250,000 in incremental cost annually for each of Drs. Schleifer and Yancopoulos. Family members or other guests may accompany our Named Officers and directors during on-demand air business travel, space permitting, so long as they cover any incremental cost related to such guests (other than with respect to the family members of Drs. Schleifer and Yancopoulos as described above). In addition, in limited circumstances personal use of on-demand air travel by our other Named Officers or directors may be permitted if authorized by the Chairman and any incremental cost is paid by the lead passenger. Any required reimbursement or other payment of the incremental cost is made to the extent permitted by applicable Federal Aviation Administration rules.
There was no unreimbursed personal use, or guest use resulting in any incremental cost to us, of Company-provided aircraft in 2015.
The Corporate Governance and Compliance Committee monitors business and any personal or guest on-demand air travel on a periodic basis.
Additional information regarding perquisites and other personal benefits provided to our Named Officers in, or with respect to, 2015 is given in the applicable footnotes to the Summary Compensation Table included in this proxy statement.
Potential Severance Benefits
Outstanding stock option award agreements (as well as outstanding restricted stock award agreements) for all employees (other than Dr. Vagelos, whose stock option awards contain change-of-control provisions consistent with those of non-employee director stock option awards, as described under "Corporate Governance – Compensation of Directors" above) include a "double trigger" provision for the acceleration of vesting of unvested stock options (or restricted stock) upon a termination by the Company without cause or by the employee for good reason within two years following a change in control. Our Chief Executive Officer has an employment agreement that provides for certain severance benefits following termination, including following death or disability, resignation following defined "good reason" events, or termination in connection with a change in control. The other Named Officers are covered by a change in control severance plan, which provides certain benefits to them and other designated officers if they are terminated in connection with a change in control. In addition, in the case of our Chief Scientific Officer, stock option and restricted stock award agreements applicable to his awards granted starting in December 2015 provide that he would have a "good reason" for terminating his employment with Regeneron upon or within two years after the occurrence of a change in control if the employment of our Chief Executive Officer has ended due to our Chief Executive Officer's involuntary termination (as defined in his employment agreement). Information regarding applicableauthorize compensation payments under this employment agreement and change in control severance plan is provided under the heading "Post-Employment Compensation" on page 66.
Except as provided in our employment agreement with our Chief Executive Officer and in our change in control severance plan, our Named Officers will forfeit any unvested time-based stock options or restricted stock upon the termination of their employment for any reason (including disability or retirement) other than death. In the event of the death of an employee, any unvested stock options held by such employee become immediately exercisable, and any shares of restricted stock will become fully vested. For information regarding unvested stock options and shares of restricted stock held by our Named Officers as of December 31, 2015, see "Outstanding Equity Awards at Fiscal Year-End" on page 64. When employees (other than our Chief Executive Officer) retire, they forfeit all unvested time-based stock options and restricted stock. For all stock options granted prior to 2007, an employee (other than our Chief Executive Officer) who retires has up to two years to exercise stock options that are vestednot deductible, such as ofwhen we believe that such payments are necessary to maintain the date of hisflexibility needed to attract talent, promote executive retention, reward performance, or her retirement. Commencing in 2007, we amended our forms of stock option agreementattain other Company objectives, or as required to allow the retired employee the remaining life of the 10-year stock option term to exercise stock options that are vested as of the date of his or her retirement.
The severance benefits provided to our Named Officers are designed to promote stability and continuity of our senior management and are intended to preserve employee morale and productivity and encourage retention in the face of the disruptive impact of an actual, threatened, or rumored change in control of the Company. The severance benefits were established following a review of comparable practices at the Company's peer companies andcomply with the adviceCompany’s contractual commitments.
84 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DISCUSSION AND ANALYSIS
We, the members of the Compensation Committee's consultant. We have no pension, deferred compensation, or retirement plans, other than our 401(k) Savings Plan described above.
59
Executive Compensation
The Compensation Committee, Report below shall not be deemed to be "soliciting material" or to be filed with the SEC or subject to Regulation 14A or 14C under the Exchange Act, or to the liabilities of Section 18 of the Exchange Act. Notwithstanding anything to the contrary set forth in any of the Company's previous filings under the Securities Act or the Exchange Act that might incorporate future filings, including this proxy statement, in whole or in part, the Compensation Committee Report below shall not be incorporated by reference into any such filings.
Compensation Committee Report
We have reviewed and discussed with management the Compensation Discussion and Analysis beginning on page 35.set forth above. Based on that review and discussion, we have recommended to the board of directors that the Compensation Discussion and Analysis be included in this proxy statement.
The Compensation Committee
Christine A. Poon, Chairperson*Charles A. BakerJoseph L. Goldstein, M.D.Chairperson
George L. Sing
Huda Y. Zoghbi, M.D.
*Ms. Poon became Chairperson of the Compensation Committee effective as of April 1, 2016.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
Compensation Committee Interlocks and Insider Participation
None of the members of the Compensation Committee is currently, or has been at any time since our formation, one of our officers or employees. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or Compensation Committee.
60
Executive Compensation

2019 EXECUTIVE COMPENSATION TABLES
The following table and accompanying footnotes provide information regarding compensation earned by, or paid to, our Named Officers (our Chief Executive Officer, our Chief Financial Officer, and ourNEOs during the last three other highest-compensated executive officers in 2015)fiscal years (other than with respect to Dr. Murphy, who qualified as an NEO for 2019 but not for 2018 or 2017).
2019 Summary Compensation Table
| A | B | C | E | F | G | I | J | |||||||
| Name and principal position | Year | Salary ($) | Stock awards ($)1 | Option awards ($)1 | Non-Equity Incentive Plan Compensation ($)2 | All Other Compensation ($)3 | Total ($) | |||||||
| Leonard S. Schleifer, M.D., Ph.D. President and Chief Executive Officer | 2019 | 1,377,100 | 4,983,226 | 11,699,586 | 3,057,162 | 338,0434 | 21,455,117 | |||||||
| 2018 | 1,330,500 | — | 21,339,913 | 2,953,710 | 896,432 | 26,520,555 | ||||||||
| 2017 | 1,285,500 | — | 21,967,210 | 2,468,160 | 787,188 | 26,508,058 | ||||||||
| George D. Yancopoulos, M.D., Ph.D. President and Chief Scientific Officer | 2019 | 1,170,500 | 4,983,226 | 11,699,586 | 2,598,510 | 212,3135 | 20,664,135 | |||||||
| 2018 | 1,130,900 | — | 21,339,913 | 2,510,598 | 399,718 | 25,381,129 | ||||||||
| 2017 | 1,092,700 | — | 21,967,210 | 2,097,984 | 141,345 | 25,299,239 | ||||||||
| Robert E. Landry Executive Vice President, Finance and Chief Financial Officer | 2019 | 730,000 | 2,840,008 | 3,526,669 | 811,395 | 23,4706 | 7,931,542 | |||||||
| 2018 | 680,000 | 4,767,500 | 3,308,184 | 581,400 | 19,535 | 9,356,619 | ||||||||
| 2017 | 618,000 | — | 3,675,566 | 482,040 | 19,130 | 4,794,736 | ||||||||
| Daniel P. Van Plew Executive Vice President and General Manager, Industrial Operations and Product Supply | 2019 | 683,100 | 2,840,008 | 3,526,669 | 759,266 | 20,4707 | 7,829,513 | |||||||
| 2018 | 660,000 | — | 7,650,147 | 677,160 | 19,915 | 9,007,222 | ||||||||
| 2017 | 560,000 | — | 7,875,030 | 524,160 | 19,410 | 8,978,600 | ||||||||
| Andrew J. Murphy, Ph.D. Executive Vice President, Research8 | 2019 | 600,000 | 4,702,308 | 3,526,669 | 666,900 | 23,4709 | 9,519,347 |
| 1 | The amounts in columns (e) and (f) reflect the respective aggregate grant date fair values (disregarding estimated forfeitures) of PSUs or RSAs (as applicable) and stock option awards granted in 2019, 2018, and 2017, respectively, pursuant to the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Valuation assumptions and methodologies used in the calculation of these amounts are included in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2019 included in the 2019 Annual Report. |
| 2 | Non-equity incentive plan compensation amounts (consisting of cash incentives paid to the NEOs in respect of the relevant year under the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan) are shown in the year in which they were accrued and earned. |
| 3 | See the subsection “Additional Compensation Information – Perquisites and Personal Benefits” below for further information. Certain 2019 perquisites and other personal benefits are quantified for each of the NEOs in the footnotes to this table below based on the actual additional cost incurred by us in providing the perquisite or other personal benefit. |
| 4 | Consists of (i) $20,724 for life insurance premiums, (ii) $44,671 for long-term disability insurance premiums, (iii) $28,857 for medical malpractice insurance premiums, (iv) $12,500 for 401(k) Savings Plan matching contributions in respect of 2019, (v) $10,970 for tax and financial planning advisory services, and (vi) $200,574 and $19,748 for personal use of Company-provided aircraft and secure car transportation/on-site residential security at Dr. Schleifer’s primary residence, respectively, in each case in accordance with our security policy (calculated as described in the subsection “Additional Compensation Information – Perquisites and Personal Benefits” below). |
| Name and principal position (a) | | Year (b) | | Salary ($) (c) | | Bonus ($)1 (d) | | Stock awards ($)2 (e) | | Option awards ($)2 (f) | | All other compensation ($)3 (i) | | Total ($) (j) | ||||||||||
| | Leonard S. Schleifer, M.D., Ph.D. | | 2015 | | 1,200,000 | | 2,880,000 | | — | | 43,307,918 | 4 | | 74,608 | 5 | | 47,462,526 | |||||||
| President and Chief | | 2014 | | 1,071,200 | | 2,142,400 | | — | | 38,644,700 | 6 | | 107,124 | | 41,965,424 | ||||||||
| Executive Officer | | 2013 | | 1,035,000 | | 2,070,000 | | — | | 33,062,325 | 7 | | 105,340 | | 36,272,665 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | George D. Yancopoulos, M.D., Ph.D. | | 2015 | | 1,020,000 | | 2,448,000 | | — | | 36,811,837 | 4 | | 22,519 | 8 | | 40,302,356 | |||||||
| President, Regeneron Laboratories | | 2014 | | 910,500 | | 1,821,040 | | — | | 32,744,746 | 6 | | 30,525 | | 35,506,811 | ||||||||
| and Chief Scientific Officer | | 2013 | | 879,800 | | 1,759,500 | | — | | 28,096,838 | 7 | | 281,455 | | 31,017,593 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Robert E. Landry | | 2015 | | 517,500 | | 465,750 | | — | | 7,246,284 | 4 | | 19,360 | 9 | | 8,248,894 | |||||||
| Senior Vice President, Finance | | 2014 | | 500,000 | | 455,000 | 10 | | — | | 6,403,138 | 6 | | 14,818 | | 7,372,956 | |||||||
| and Chief Financial Officer* | | 2013 | | 144,231 | 11 | | 50,000 | 12 | | 1,363,500 | 13 | | 11,054,274 | 7 | | 1,130 | | 12,613,135 | |||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Neil Stahl, Ph.D. | | 2015 | | 550,000 | | 594,000 | | — | | 17,209,995 | 4 | | 20,515 | 14 | | 18,374,510 | |||||||
| Executive Vice President, | | 2014 | | 493,500 | | 532,980 | | — | | 15,207,420 | 6 | | 20,215 | | 16,254,115 | ||||||||
| Research & Development | | 2013 | | 476,800 | | 386,208 | | — | | 13,474,403 | 7 | | 17,235 | | 14,354,646 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Robert J. Terifay | | 2015 | | 532,100 | | 574,668 | | — | | 10,029,459 | 4 | | 20,515 | 14 | | 11,156,742 | |||||||
| Executive Vice President, | | 2014 | | 465,800 | | 377,298 | | — | | 7,533,104 | 6 | | 20,125 | | 8,396,327 | ||||||||
| Commercial | | 2013 | | 450,000 | | 364,500 | | — | | 6,847,486 | 7 | | 17,145 | | 7,679,131 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
*Mr. Landry joined the Company in September 2013.
1Bonuses are shown in the year in which they were accrued and earned.
2The amounts in column (e) and (f) reflect the respective aggregate grant date fair values (disregarding estimated forfeitures) of stock and option awards granted in 2015, 2014, and 2013, respectively, pursuant to the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan or the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan. Assumptions used in the calculation of these amounts are included in Note 14 to the Company's audited financial statements for the fiscal year ended December 31, 2015 included in the 2015 Annual Report. The 2013, 2014, and 2015 annual option awards reflect a double-digit percentage decrease in the number of shares underlying the awards, in each case as compared to the prior year (other
than Mr. Terifay's 2015 award, which remained at the 2014 level due to his promotion to Executive Vice President, Commercial). Mr. Landry did not receive an annual option award in 2013; his 2013 option award was granted pursuant to his offer letter with the Company in connection with the commencement of his employment.
3See "Compensation Discussion and Analysis – Section 4 – Elements of Executive Compensation – Perquisites and Other Personal Benefits" for further information. Certain 2015 perquisites and other personal benefits are quantified for each of the Named Officers in the footnotes to this table below based on the actual additional cost incurred by us in providing the perquisite or other personal benefit.
4Reflects the aggregate grant date fair value of time-based option awards granted in 2015.
| 5 | Consists of (i) $12,500 for 401(k) Savings Plan matching contributions in respect of 2019, (ii) $10,970 for tax and financial planning advisory services, and (iii) $184,546 and $4,297 for personal use of Company-provided aircraft and secure car transportation/on-site residential security at Dr. Yancopoulos’s primary residence, respectively, in each case in accordance with our security policy (calculated as described in the subsection “Additional Compensation Information –Perquisites and Personal Benefits” below). |
| 6 | Consists of (i) $12,500 for 401(k) Savings Plan matching contributions in respect of 2019 and (ii) $10,970 for tax and financial planning advisory services. |
| 7 | Consists of (i) $9,500 for 401(k) Savings Plan matching contributions in respect of 2019 and (ii) $10,970 for tax and financial planning advisory services. |
| 8 | Dr. Murphy qualified as an NEO for 2019 but not for 2018 or 2017. |
| 9 | Consists of $12,500 for 401(k) Savings Plan matching contributions in respect of 2019 and (ii) $10,970 for tax and financial planning advisory services. |
61
Executive Compensation
5Includes (i) $1,655 for life insurance premiums, (ii) $23,961 for long-term disability insurance premiums, (iii) $13,889 for medical malpractice insurance premiums, (iv) $10,600 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016, (v) $9,915 for tax and financial planning advisory services, and (vi) $14,200 for personal use of secure car transportation in accordance with our security policy.
6Reflects the aggregate grant date fair value of time-based option awards granted in 2014.
7Reflects the aggregate grant date fair value of time-based option awards granted in 2013. In the case of Mr. Landry, such option award was granted pursuant to his offer letter with the Company in connection with the commencement of his employment.
8Consists of (i) $10,600 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016, (ii) $9,915 for tax and financial planning advisory services, and (iii) $2,004 for personal use of secure car transportation in accordance with our security policy.
9Consists of (i) $10,600 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016 and (ii) $8,760 for tax and financial planning advisory services.
10Includes the second installment in the amount of $50,000 of Mr. Landry's $100,000 sign-on bonus (paid in 2014).
11Represents Mr. Landry's base salary paid from September 9, 2013 (the starting date of Mr. Landry's employment with the Company) to the end of 2013.
12Consists of the first installment of Mr. Landry's $100,000 sign-on bonus (paid in 2013).
13Reflects the aggregate grant date fair value of restricted stock granted to Mr. Landry pursuant to his offer letter with the Company in connection with the commencement of his employment.
14Consists of (i) $10,600 for 401(k) Savings Plan matching contributions in respect of 2015 paid in February 2016 and (ii) $9,915 for tax and financial planning advisory services.
62
Executive Compensation
 | COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD |
2019 Grants of Plan-Based Awards
The following table and explanatory footnotes provide information regarding eachthe annual cash incentive and equity awardawards granted to our Named OfficersNEOs during 2015. There were no non-equity incentive plan awards granted in 2015.2019.
Grants of Plan-Based Awards
| Name (a) | | Grant date (b) | | All other stock awards: number of shares of stock or units (#) (i) | | All other option awards: number of securities underlying options (#) (j) | | Exercise or base price of option awards ($/Sh)1 (k) | | Closing price of Company common stock on grant date ($/Sh)1 (l) | | Grant date fair value of stock and option awards ($)2 (l) | |||||||
| Leonard S. Schleifer, M.D., Ph.D. | | 12/16/2015 | 3 | | — | | 172,723 | | 555.67 | | 559.67 | | 43,307,918 | ||||||
| | | | | | | | | | | | | | | | | | | | |
| George D. Yancopoulos, M.D., Ph.D. | | 12/16/2015 | 3 | | — | | 146,815 | | 555.67 | | 559.67 | | 36,811,837 | ||||||
| | | | | | | | | | | | | | | | | | | | |
| Robert E. Landry | | 12/16/2015 | 3 | | — | | 28,900 | | 555.67 | | 559.67 | | 7,246,284 | ||||||
| | | | | | | | | | | | | | | | | | | | |
| Neil Stahl, Ph.D. | | 12/16/2015 | 3 | | — | | 68,638 | | 555.67 | | 559.67 | | 17,209,995 | ||||||
| | | | | | | | | | | | | | | | | | | | |
| Robert J. Terifay | | 12/16/2015 | 3 | | — | | 40,000 | | 555.67 | | 559.67 | | 10,029,459 | ||||||
| | | | | | | | | | | | | | | | | | | | |
1These options have an exercise price equal to the average of the high and low sales price per share of the Company's common stock on the date of grant. Therefore, the closing price of our common stock on the grant date may be higher or lower than the exercise price of these options.
2The amounts in this column represent the grant date fair value of the awards made pursuant to the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The assumptions used in the calculation of these amounts are included in Note 14 to the Company's
audited financial statements for the fiscal year ended December 31, 2015 included in the 2015 Annual Report.
3The Named Officer received a non-qualified stock option award that vests at a rate of 25% per year over the first four years of the ten-year option term.
63
Executive Compensation
| A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Estimated Possible Payouts Under Non-Equity Incentive Plan Awards1 | Estimated Possible Payouts Under Equity Incentive Plan Awards2 | All other stock awards: number of shares of | All other option awards: number of securities | Exercise or base price of | Closing price of Company common | Grant date fair value of stock | ||||||||||||||||||
| Name | Grant date | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | stock or units (#) | Underlying options (#) | Option awards ($/Sh)3 | stock on grant date ($/Sh)3 | and option awards ($)4 | ||||||||||||
| Leonard S. Schleifer, M.D., Ph.D. | — | — | 1,652,520 | 3,305,040 | — | — | — | — | — | — | — | — | ||||||||||||
| 12/11/2019 | 5 | — | — | — | — | — | — | — | 81,278 | 372.46 | 373.53 | 11,699,586 | ||||||||||||
| 12/11/2019 | — | — | — | 5,590 | 11,180 | 25,155 | — | — | — | — | 4,983,226 | |||||||||||||
| George D. Yancopoulos, M.D., Ph.D. | — | — | 1,404,600 | 2,809,200 | — | — | — | — | — | — | — | — | ||||||||||||
| 12/11/2019 | 5 | — | — | — | — | — | — | — | 81,278 | 372.46 | 373.53 | 11,699,586 | ||||||||||||
| 12/11/2019 | — | — | — | 5,590 | 11,180 | 25,155 | — | — | — | — | 4,983,226 | |||||||||||||
| Robert E. Landry | — | — | 474,500 | 854,100 | — | — | — | — | — | — | — | — | ||||||||||||
| 12/11/2019 | 5 | — | — | — | — | — | — | — | 24,500 | 372.46 | 373.53 | 3,526,669 | ||||||||||||
| 12/11/2019 | 6 | — | — | — | — | — | — | 2,625 | — | — | — | 977,708 | ||||||||||||
| 12/11/2019 | 7 | — | — | — | — | — | — | 5,000 | — | — | — | 1,862,300 | ||||||||||||
| Daniel P. Van Plew | — | — | 444,015 | 799,227 | — | — | — | — | — | — | — | — | ||||||||||||
| 12/11/2019 | 5 | — | — | — | — | — | — | — | 24,500 | 372.46 | 373.53 | 3,526,669 | ||||||||||||
| 12/11/2019 | 6 | — | — | — | — | — | — | 2,625 | — | — | — | 977,708 | ||||||||||||
| 12/11/2019 | 7 | — | — | — | — | — | — | 5,000 | — | — | — | 1,862,300 | ||||||||||||
| Andrew J. Murphy, Ph.D. | — | — | 390,000 | 702,000 | — | — | — | — | — | — | — | — | ||||||||||||
| 12/11/2019 | 5 | — | — | — | — | — | — | — | 24,500 | 372.46 | 373.53 | 3,526,669 | ||||||||||||
| 12/11/2019 | 6 | — | — | — | — | — | — | 2,625 | — | — | — | 977,708 | ||||||||||||
| 12/11/2019 | 7 | — | — | — | — | — | — | 10,000 | — | — | — | 3,724,600 | ||||||||||||
| Cash incentive awards under the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan. The actual cash incentive awards earned in respect of 2019 and paid out in January 2020 are reported as “Non-Equity Incentive Plan Compensation” in the Summary Compensation Table above. | |
| The amounts in this column represents the threshold, target, and maximum number of shares of common stock that may be earned by the NEO in respect of the PSUs granted to the NEO in 2019. | |
| 3 | These options have an exercise price equal to the average of the high and low sales price per share of the Company’s common stock on the date of grant. Therefore, the closing price of our common stock on the grant date may be higher or lower than the exercise price of these options. |
| 4 | The amounts in this column represent the grant date fair value (disregarding estimated forfeitures) of the awards made pursuant to the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The valuation assumptions and methodologies used in the calculation of these amounts are included in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2019 included in the 2019 Annual Report. |
| 5 | The NEO received a non-qualified stock option award that vests subject to continued employment at |
| 6 | The NEO received an annual RSA that vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 7 | The NEO received a special RSA that vests 100% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 87 / 87 |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
Outstanding Equity Awards at 2019 Fiscal Year-End
The following table and explanatory footnotes provide information regarding unexercised stock options and unvested restricted stock awardsPSUs or RSAs (as applicable) held by our Named OfficersNEOs as of December 31, 2015.2019.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| | Option Awards | | Stock Awards | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | Number of securities underlying unexercised options (#) exercisable (b) | | Number of securities underlying unexercised options (#) unexercisable (c) | | Equity incentive plan awards: number of securities underlying unexercised unearned options (#) (d) | | Option exercise price ($) (e) | | Option expiration date (f) | | Number of shares or units of stock that have not vested (#) (g) | | Market value of shares or units of stock that have not vested ($)5 (h) | | Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) (i) | | Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) (j) | ||||||||||
Leonard S. Schleifer, M.D., Ph.D. | | — | | 172,723 | 1 | | — | | 555.67 | | 12/16/2025 | | — | | — | | — | | — | |||||||||
| | 50,801 | | 152,403 | 2 | | — | | 399.66 | | 12/16/2024 | | — | | — | | — | | — | |||||||||
| | 119,532 | | 119,531 | 3 | | — | | 270.43 | | 12/13/2023 | | — | | — | | — | | — | |||||||||
| | 210,938 | | 70,312 | 4 | | — | | 179.13 | | 12/14/2022 | | — | | — | | — | | — | |||||||||
| | 160,000 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 240,000 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 125,000 | | — | | — | | 30.63 | | 12/14/2020 | | — | | — | | — | | — | ||||||||||
| | 187,500 | | — | | — | | 30.63 | | 12/14/2020 | | — | | — | | — | | — | ||||||||||
| | 187,500 | | — | | — | | 21.25 | | 12/18/2019 | | — | | — | | — | | — | ||||||||||
| | 125,000 | | — | | — | | 21.25 | | 12/18/2019 | | — | | — | | — | | — | ||||||||||
| | 125,000 | | — | | — | | 16.80 | | 12/17/2018 | | — | | — | | — | | — | ||||||||||
| | 187,500 | | — | | — | | 16.80 | | 12/17/2018 | | — | | — | | — | | — | ||||||||||
| | 250,000 | | — | | — | | 21.92 | | 12/17/2017 | | — | | — | | — | | — | ||||||||||
| | 250,000 | | — | | — | | 20.32 | | 12/18/2016 | | — | | — | | — | | — | ||||||||||
TOTAL | | 2,218,771 | | 514,969 | | | | | | | | | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
George D. Yancopoulos, M.D., Ph.D. | | — | | 146,815 | 1 | | — | | 555.67 | | 12/16/2025 | | — | | — | | — | | — | |||||||||
| | 43,181 | | 129,542 | 2 | | — | | 399.66 | | 12/16/2024 | | — | | — | | — | | — | |||||||||
| | 101,602 | | 101,602 | 3 | | — | | 270.43 | | 12/13/2023 | | — | | — | | — | | — | |||||||||
| | 179,298 | | 59,765 | 4 | | — | | 179.13 | | 12/14/2022 | | — | | — | | — | | — | |||||||||
| | 158,079 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 240,000 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 96,736 | | — | | — | | 30.63 | | 12/14/2020 | | — | | — | | — | | — | ||||||||||
| | 150,000 | | — | | — | | 30.63 | | 12/14/2020 | | — | | — | | — | | — | ||||||||||
| | 150,000 | | — | | — | | 21.25 | | 12/18/2019 | | — | | — | | — | | — | ||||||||||
| | 95,295 | | — | | — | | 21.25 | | 12/18/2019 | | — | | — | | — | | — | ||||||||||
| | 94,048 | | — | | — | | 16.80 | | 12/17/2018 | | — | | — | | — | | — | ||||||||||
| | 150,000 | | — | | — | | 16.80 | | 12/17/2018 | | — | | — | | — | | — | ||||||||||
| | 195,438 | | — | | — | | 21.92 | | 12/17/2017 | | — | | — | | — | | — | ||||||||||
| | 195,079 | | — | | — | | 20.32 | | 12/18/2016 | | — | | — | | — | | — | ||||||||||
| | — | | — | | — | | — | | — | | 500,000 | 6 | | 542.87 | | — | | — | |||||||||
TOTAL | | 1,848,756 | | 437,724 | | | | | | | | 500,000 | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Robert E. Landry | | — | | 28,900 | 1 | | — | | 555.67 | | 12/16/2025 | | — | | — | | — | | — | |||||||||
| | 8,500 | | 25,500 | 2 | | — | | 399.66 | | 12/16/2024 | | — | | — | | — | | — | |||||||||
| | 26,000 | | 40,000 | 7 | | — | | 272.70 | | 9/9/2023 | | — | | — | | — | | — | |||||||||
| | — | | — | | — | | — | | — | | 5,000 | 8 | | 542.87 | | — | | — | |||||||||
TOTAL | | 34,500 | | 94,400 | | | | | | | | 5,000 | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| A | B | C | D | E | F | G | H | I | J | |||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Equity | ||||||||||||||||||||||||||||||||||||
| Equity | Equity | incentive | ||||||||||||||||||||||||||||||||||
| incentive | incentive | plan awards: | ||||||||||||||||||||||||||||||||||
| plan awards: | plan awards: | market or | ||||||||||||||||||||||||||||||||||
| Number of | Number of | number of | number of | payout value | ||||||||||||||||||||||||||||||||
| securities | securities | securities | Number of | Market value | unearned | of unearned | ||||||||||||||||||||||||||||||
| underlying | underlying | underlying | shares or | of shares or | shares, units | shares, units | ||||||||||||||||||||||||||||||
| unexercised | unexercised | unexercised | units of stock | units of stock | or other rights | or other rights | ||||||||||||||||||||||||||||||
| options | options | unearned | Option | Option | that have not | that have not | that have not | that have not | ||||||||||||||||||||||||||||
| exercisable | unexercisable | options | exercise price | expiration | vested | vested | vested | vested | ||||||||||||||||||||||||||||
| Name | (#) | (#) | (#) | ($) | date | (#) | ($) | (#) | ($) | |||||||||||||||||||||||||||
| — | 81,278 | 1 | — | 372.46 | 12/11/2029 | — | — | — | — | |||||||||||||||||||||||||||
| 32,254 | 96,759 | 2 | — | 381.40 | 12/12/2028 | — | — | — | — | |||||||||||||||||||||||||||
| 69,738 | 69,736 | 3 | — | 378.98 | 12/12/2027 | — | — | — | — | |||||||||||||||||||||||||||
| 110,112 | 36,703 | 4 | — | 381.92 | 12/16/2026 | — | — | — | — | |||||||||||||||||||||||||||
| 172,723 | — | — | 555.67 | 12/16/2025 | — | — | — | — | ||||||||||||||||||||||||||||
| Leonard S. Schleifer, M.D., Ph.D. | 203,204 | — | — | 399.66 | 12/16/2024 | — | — | — | — | |||||||||||||||||||||||||||
| 239,063 | — | — | 270.43 | 12/13/2023 | — | — | — | — | ||||||||||||||||||||||||||||
| 281,250 | — | — | 179.13 | 12/14/2022 | — | — | — | — | ||||||||||||||||||||||||||||
| 160,000 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 240,000 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 125,000 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| 187,500 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| — | — | — | — | — | — | — | 5,590 | 5 | 2,098,933 | 6 | ||||||||||||||||||||||||||
| TOTAL | 1,820,844 | 284,476 | 5,590 | |||||||||||||||||||||||||||||||||
| — | 81,278 | 1 | — | 372.46 | 12/11/2029 | — | — | — | — | |||||||||||||||||||||||||||
| 32,254 | 96,759 | 2 | — | 381.40 | 12/12/2028 | — | — | — | — | |||||||||||||||||||||||||||
| 69,738 | 69,736 | 3 | — | 378.98 | 12/12/2027 | — | — | — | — | |||||||||||||||||||||||||||
| 110,112 | 36,703 | 4 | — | 381.92 | 12/16/2026 | — | — | — | — | |||||||||||||||||||||||||||
| 146,815 | — | — | 555.67 | 12/16/2025 | — | — | — | — | ||||||||||||||||||||||||||||
| 172,723 | — | — | 399.66 | 12/16/2024 | — | — | — | — | ||||||||||||||||||||||||||||
| George D. Yancopoulos, M.D., Ph.D. | 203,204 | — | — | 270.43 | 12/13/2023 | — | — | — | — | |||||||||||||||||||||||||||
| 239,063 | — | — | 179.13 | 12/14/2022 | — | — | — | — | ||||||||||||||||||||||||||||
| 158,079 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 240,000 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 96,736 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| 150,000 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| — | — | — | — | — | — | — | 5,590 | 5 | 2,098,933 | 6 | ||||||||||||||||||||||||||
| TOTAL | 1,618,724 | 284,476 | 5,590 | |||||||||||||||||||||||||||||||||
| — | 24,500 | 1 | — | 372.46 | 12/11/2029 | — | — | — | — | |||||||||||||||||||||||||||
| 5,000 | 15,000 | 2 | — | 381.40 | 12/12/2028 | — | — | — | — | |||||||||||||||||||||||||||
| 11,669 | 11,668 | 3 | — | 378.98 | 12/12/2027 | — | — | — | — | |||||||||||||||||||||||||||
| 18,424 | 6,141 | 4 | — | 381.92 | 12/16/2026 | — | — | — | — | |||||||||||||||||||||||||||
| 28,900 | — | — | 555.67 | 12/16/2025 | — | — | — | — | ||||||||||||||||||||||||||||
| Robert E. Landry | 34,000 | — | — | 399.66 | 12/16/2024 | — | — | — | — | |||||||||||||||||||||||||||
| 57,000 | — | — | 272.70 | 9/9/2023 | — | — | — | — | ||||||||||||||||||||||||||||
| — | — | — | — | — | 2,625 | 7 | 985,635 | 6 | — | — | ||||||||||||||||||||||||||
| — | — | — | — | — | 5,000 | 8 | 1,877,400 | 6 | — | — | ||||||||||||||||||||||||||
| — | — | — | — | — | 12,500 | 9 | 4,693,500 | 6 | — | — | ||||||||||||||||||||||||||
| TOTAL | 154,993 | 57,309 | 20,125 | |||||||||||||||||||||||||||||||||
64
Executive Compensation
| | Option Awards | | Stock Awards | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | Number of securities underlying unexercised options (#) exercisable (b) | | Number of securities underlying unexercised options (#) unexercisable (c) | | Equity incentive plan awards: number of securities underlying unexercised unearned options (#) (d) | | Option exercise price ($) (e) | | Option expiration date (f) | | Number of shares or units of stock that have not vested (#) (g) | | Market value of shares or units of stock that have not vested ($)5 (h) | | Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) (i) | | Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) (j) | ||||||||||
Neil Stahl, Ph.D. | | — | | 68,638 | 1 | | — | | 555.67 | | 12/16/2025 | | — | | — | | — | | — | |||||||||
| | 20,188 | | 60,562 | 2 | | — | | 399.66 | | 12/16/2024 | | — | | — | | — | | — | |||||||||
| | 47,500 | | 47,500 | 3 | | — | | 270.43 | | 12/13/2023 | | — | | — | | — | | — | |||||||||
| | 4,527 | | — | | — | | 268.40 | | 12/17/2017 | | — | | — | | — | | — | ||||||||||
| | 84,375 | | 28,125 | 4 | | — | | 179.13 | | 12/14/2022 | | — | | — | | — | | — | |||||||||
| | 6,017 | | — | | — | | 145.70 | | 12/17/2017 | | — | | — | | — | | — | ||||||||||
| | 48,079 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 75,000 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 46,736 | | — | | — | | 30.63 | | 12/14/2020 | | — | | — | | — | | — | ||||||||||
| | 20,295 | | — | | — | | 21.25 | | 12/18/2019 | | — | | — | | — | | — | ||||||||||
TOTAL | | 352,717 | | 204,825 | | | | | | | | | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Robert J. Terifay | | — | | 40,000 | 1 | | — | | 555.67 | | 12/16/2025 | | — | | — | | — | | — | |||||||||
| | 10,000 | | 30,000 | 2 | | — | | 399.66 | | 12/16/2024 | | — | | — | | — | | — | |||||||||
| | 25,000 | | 25,000 | 3 | | — | | 270.43 | | 12/13/2023 | | — | | — | | — | | — | |||||||||
| | 56,250 | | 18,750 | 4 | | — | | 179.13 | | 12/14/2022 | | — | | — | | — | | — | |||||||||
| | 1,921 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 30,579 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
| | 48,750 | | — | | — | | 52.03 | | 12/16/2021 | | — | | — | | — | | — | ||||||||||
TOTAL | | 172,500 | | 113,750 | | | | | | | | | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
1This stock option award was granted to the Named Officer on December 16, 2015 and vests at a rate of 25% per year over the first four years of the option term.
2This stock option award was granted to the Named Officer on December 16, 2014 and vests at a rate of 25% per year over the first four years of the option term.
3This stock option award was granted to the Named Officer on December 13, 2013 and vests at a rate of 25% per year over the first four years of the option term.
4This stock option award was granted to the Named Officer on December 14, 2012 and vests at a rate of 25% per year over the first four years of the option term.
5Reflects the closing price per share of the Company's common stock on the NASDAQ Global Select Market on December 31, 2015.
6This restricted stock award was granted to the Named Officer on June 27, 2012 and vests 100% on December 17, 2017, subject to the Named Officer's continued employment.
7This stock option award was granted to the Named Officer on September 9, 2013 and vests at a rate of 25% per year over the first four years of the option term.
8This restricted stock award was granted to the Named Officer on September 9, 2013 and vests 100% on the fifth anniversary of the date of grant, subject to the Named Officer's continued employment.
65
Executive Compensation
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
| A | B | C | D | E | F | G | H | I | J | |||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Equity | ||||||||||||||||||||||||||||||||||||
| Equity | Equity | incentive | ||||||||||||||||||||||||||||||||||
| incentive | incentive | plan awards: | ||||||||||||||||||||||||||||||||||
| plan awards: | plan awards: | market or | ||||||||||||||||||||||||||||||||||
| Number of | Number of | number of | number of | payout value | ||||||||||||||||||||||||||||||||
| securities | securities | securities | Number of | Market value | unearned | of unearned | ||||||||||||||||||||||||||||||
| underlying | underlying | underlying | shares or | of shares or | shares, units | shares, units | ||||||||||||||||||||||||||||||
| unexercised | unexercised | unexercised | units of stock | units of stock | or other rights | or other rights | ||||||||||||||||||||||||||||||
| options | options | unearned | Option | Option | that have not | that have not | that have not | that have not | ||||||||||||||||||||||||||||
| exercisable | unexercisable | options | exercise price | expiration | vested | vested | vested | vested | ||||||||||||||||||||||||||||
| Name | (#) | (#) | (#) | ($) | date | (#) | ($) | (#) | ($) | |||||||||||||||||||||||||||
| — | 24,500 | 1 | — | 372.46 | 12/11/2029 | — | — | — | — | |||||||||||||||||||||||||||
| 11,563 | 34,687 | 2 | — | 381.40 | 12/12/2028 | — | — | — | — | |||||||||||||||||||||||||||
| 25,000 | 25,000 | 3 | — | 378.98 | 12/12/2027 | — | — | — | — | |||||||||||||||||||||||||||
| 25,500 | 8,500 | 4 | — | 381.92 | 12/16/2026 | — | — | — | — | |||||||||||||||||||||||||||
| 40,000 | — | — | 555.67 | 12/16/2025 | — | — | — | — | ||||||||||||||||||||||||||||
| 40,000 | — | — | 399.66 | 12/16/2024 | — | — | — | — | ||||||||||||||||||||||||||||
| Daniel P. Van Plew | 50,000 | — | — | 270.43 | 12/13/2023 | — | — | — | — | |||||||||||||||||||||||||||
| 75,000 | — | — | 179.13 | 12/14/2022 | — | — | — | — | ||||||||||||||||||||||||||||
| 52,500 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 33,079 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 32,735 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| — | — | — | — | — | 2,625 | 7 | 985,635 | 6 | — | — | ||||||||||||||||||||||||||
| — | — | — | — | — | 5,000 | 8 | 1,877,400 | 6 | — | — | ||||||||||||||||||||||||||
| TOTAL | 385,377 | 92,687 | 7,625 | |||||||||||||||||||||||||||||||||
| — | 24,500 | 1 | — | 372.46 | 12/11/2029 | — | — | — | — | |||||||||||||||||||||||||||
| 6,250 | 18,750 | 2 | — | 381.40 | 12/12/2028 | — | — | — | — | |||||||||||||||||||||||||||
| 25,000 | 25,000 | 3 | — | 378.98 | 12/12/2027 | — | — | — | — | |||||||||||||||||||||||||||
| 25,500 | 8,500 | 4 | — | 381.92 | 12/16/2026 | — | — | — | — | |||||||||||||||||||||||||||
| 35,000 | — | — | 555.67 | 12/16/2025 | — | — | — | — | ||||||||||||||||||||||||||||
| 40,000 | — | — | 399.66 | 12/16/2024 | — | — | — | — | ||||||||||||||||||||||||||||
| 40,000 | — | — | 270.43 | 12/13/2023 | — | — | — | — | ||||||||||||||||||||||||||||
| Andrew J. Murphy, Ph.D. | 40,000 | — | — | 179.13 | 12/14/2022 | — | — | — | — | |||||||||||||||||||||||||||
| 33,079 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 1,921 | — | — | 52.03 | 12/16/2021 | — | — | — | — | ||||||||||||||||||||||||||||
| 26,736 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| 3,264 | — | — | 30.63 | 12/14/2020 | — | — | — | — | ||||||||||||||||||||||||||||
| — | — | — | — | — | 2,625 | 7 | 985,635 | 6 | — | — | ||||||||||||||||||||||||||
| — | — | — | — | — | 10,000 | 8 | 3,754,800 | 6 | — | — | ||||||||||||||||||||||||||
| — | — | — | — | — | 15,000 | 9 | 5,632,200 | 6 | — | — | ||||||||||||||||||||||||||
| TOTAL | 276,750 | 76,750 | 27,625 | |||||||||||||||||||||||||||||||||
| 1 | This stock option award was granted to the NEO on December 11, 2019 and vests at a rate of 25% per year over the first four years of the option term. |
| 2 | This stock option award was granted to the NEO on December 12, 2018 and vests at a rate of 25% per year over the first four years of the option term. |
| 3 | This stock option award was granted to the NEO on December 12, 2017 and vests at a rate of 25% per year over the first four years of the option term. |
| 4 | This stock option award was granted to the NEO on December 16, 2016 and vests at a rate of 25% per year over the first four years of the option term. |
| 5 | This PSU award was granted to the NEO on December 11, 2019 and has a five-year performance period from the date of grant. Based on performance as of December 31, 2019 in accordance with SEC rules, the number of PSUs shown in this table assumes a threshold level of payout. |
| 6 | Reflects the closing price of $375.48 per share of the Company’s common stock on the Nasdaq Global Select Market on December 31, 2019. |
| 7 | This RSA was granted to the NEO on December 11, 2019 and vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 8 | This RSA was granted to the NEO on December 11, 2019 and vests 100% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 9 | This RSA was granted to the NEO on December 12, 2018 and vests 100% on the fifth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 89 / 89 |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
2019 Option Exercises and Stock Vested
The following table and explanatory footnotes provide information with regard to amounts realized by our Named OfficersNEOs during 20152019 as a result of the exercise of stock options or the vesting of restricted stock awards.RSAs.
Option Exercises and Stock Vested
| A | B | C | D | E | ||||||||||||
| Option awards | Stock awards | |||||||||||||||
| Number of shares | Value realized | Number of shares | Value realized | |||||||||||||
| Name | acquired on exercise | on exercise | acquired on vesting | on vesting | ||||||||||||
| (a) | (#) | ($)1 | (#) | ($) | ||||||||||||
| Leonard S. Schleifer, M.D., Ph.D. | 312,500 | 111,224,257 | — | — | ||||||||||||
| George D. Yancopoulos, M.D., Ph.D. | 245,295 | 87,302,943 | — | — | ||||||||||||
| Robert E. Landry | — | — | — | — | ||||||||||||
| Daniel P. Van Plew | — | — | — | — | ||||||||||||
| Andrew J. Murphy, Ph.D. | 30,000 | 7,929,983 | — | — | ||||||||||||
| | Option awards | | Stock awards | |||||||||
| | | | | | | | | | | | | | |
Name | | Number of shares acquired on exercise (#) (b) | | Value realized on exercise ($)1 (c) | | Number of shares acquired on vesting (#) (d) | | Value realized on vesting ($) (e) | |||||
Leonard S. Schleifer, M.D., Ph.D. | | 7,226 | | 749,192 | | — | | — | |||||
George D. Yancopoulos, M.D., Ph.D. | | 184,739 | | 101,049,378 | | — | | — | |||||
Robert E. Landry | | 14,000 | | 3,048,560 | | — | | — | |||||
Neil Stahl, Ph.D. | | 101,921 | | 54,901,992 | | — | | — | |||||
Robert J. Terifay | | 81,250 | | 37,906,863 | | — | | — | |||||
| | | | | | | | | | | | | | |
1Amounts reflect the difference between the exercise price of the option(s) and the average of the high and low sales price per share of the Company's common stock on the NASDAQ Global Select Market on the exercise date(s).
| 1 | Amounts reflect the difference between the exercise price of the option(s) and the average of the high and low sales price per share of the Company’s common stock on the NASDAQ Global Select Market on the exercise date(s). |
Post-Employment CompensationPOST-EMPLOYMENT COMPENSATION
As discussed under "Section 4 – Elements of Executivein “Compensation Dashboard—Additional Compensation – Information—Potential Severance Benefits" on page 59,Payments,” our Named OfficersNEOs are entitled to certain severance benefits upon the voluntary or involuntary termination of their employment. We provide additional information regarding the severance benefits available to our Named OfficersNEOs in the tables on pages 67 and 69.set out below in this subsection. For our Chief Executive Officer,CEO, the table shows the amounts payable under his employment agreement upon his involuntary or not-for-cause termination, termination in connection with a corporate change of control, and in the event of his disability or death. For the other Named Officers,NEOs, the table shows their post-termination compensation arrangements under our change in control severance plan upon an involuntary or not-for-cause termination in connection with a corporate change of control.
Leonard S. Schleifer, M.D., Ph.D., Employment Agreement
We entered into an employment agreement with our Chief Executive Officer,CEO, Dr. Schleifer, effective as of December 20, 2002, providing for his employment with the Company through December 31, 2003 and continuing thereafter on a year-by-year basis. On November 14, 2008, this employment agreement was amended and restated to bring the employment agreement into compliance with Section 409A of the
Internal Revenue Code. Pursuant to this agreement, we agreed that in the event that Dr. Schleifer'sSchleifer’s employment is terminated by us other than for cause (as defined in the agreement) or is terminated by Dr. Schleifer for good reason (as defined in the agreement to include specified acts of constructive termination, together called an "involuntary termination"“involuntary termination”), we will pay Dr. Schleifer an amount equal to 125% of the sum of his base salary plus his average bonuscash incentive paid over the prior three years. This amount will be paid in a lump sumlump-sum severance payment. In addition, we will continue to provide Dr. Schleifer and his dependents medical, dental, and life insurance benefits for eighteen18 months. Subject to the discussion in the following paragraph, in the event that Dr. Schleifer'sSchleifer’s employment is terminatedterminates for any reason other than for cause, (i) all of his unvested stock options will continue to vest in accordance with the terms of the applicable award grant and he will be entitled to exercise the stock options throughout their original term, which is generally ten years from the date of grant.grant, and (ii) all of his unvested PSUs will remain outstanding, and vesting and forfeiture shall be determined in the manner set forth in the applicable award agreement, without regard to such termination of employment.
Upon an involuntary termination (i.e., a termination by the Company without cause or by Dr. Schleifer for good reason, each as defined in the agreement) within three years after a change of control of the Company or within three months prior to such a change of control, we will pay Dr. Schleifer an amount equal to three times the sum of his annual base salary plus his average bonuscash incentive over the prior three years. This amount will be paid in a lump sumlump-sum severance payment. In addition, we will continue to provide Dr. Schleifer and his dependents medical, dental, and life insurance benefits for thirty-six36 months. Upon such an involuntary termination in
66
Executive Compensation
connection with a change of control, Dr. Schleifer'sSchleifer’s outstanding stock options will vest immediately and remain exercisable throughout their original term, which is generally ten years from the date of grant. In addition, pursuant to the terms of his PSU award agreement, any PSUs
90 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
that vest upon a change of control (as a result of performance exceeding the relevant TSR goal for the period from the grant date to the date of the change of control) will become deliverable to Dr. Schleifer upon the earlier of (x) the five-year anniversary of the grant date or (y) a termination of Dr. Schleifer’s employment by the Company without cause or by Dr. Schleifer for good reason, in each case within two years after such a change of control. If aggregate severance payments to Dr. Schleifer in connection with a change of control exceed certain thresholds set forth in the Internal Revenue Code, then we will pay him an additional amount to cover any resulting excise tax obligations, unless the excise taxes could be eliminated by reducing Dr. Schleifer'sSchleifer’s cash severance payments and benefits under the agreement by less than ten
percent,10%, in which case such benefits and payments will be reduced accordingly.
The following table reflects the potential payments to our Chief Executive OfficerCEO under his employment agreement upon hisassuming a termination effective December 31, 2015,2019 under different scenarios including(including following a change of control,control), as well as upon death or disability. The information in the table below is based on the assumptions set forth in the footnotes to the table; actual values and amounts may differ from those presented below.
Potential Severance Payments under Dr. Schleifer'sSchleifer’s Employment Agreement
| Value of | ||||||||||||||||||||||||||||
| Accelerated | ||||||||||||||||||||||||||||
| Cash | Benefits | Death | Disability | Stock Options/ | Cutback/ | Total | ||||||||||||||||||||||
| Severance | Continuation | Benefits4 | Benefits | PSUs | Gross-up6 | Amount | ||||||||||||||||||||||
| Involuntary Termination | $ | 11,788,770 | 2 | $ | 265,772 | 3 | — | — | $ | 245,460 | 5 | — | $ | 12,300,002 | ||||||||||||||
| Following a Change of Control1 | ||||||||||||||||||||||||||||
| Involuntary Termination | $4,911,988 | 7 | $129,258 | 8 | — | — | — | — | $5,041,246 | |||||||||||||||||||
| Death | — | $98,172 | 9 | — | — | — | 10 | — | $98,172 | |||||||||||||||||||
| Disability | — | $129,258 | 8 | — | $ | 722,978 | 11 | — | — | $852,236 | ||||||||||||||||||
| 1 | For purposes of these calculations, (i) we used Dr. Schleifer’s 2019 base salary and the annual cash incentives paid to Dr. Schleifer for performance in 2016, 2017, and 2018, respectively; (ii) we assumed that Dr. Schleifer received his annual cash incentive that was earned in 2019 and paid in 2020 (described in the Summary Compensation Table above); (iii) we took into consideration, for purposes of determining whether Dr. Schleifer was entitled to receive a gross-up payment under the terms of his employment agreement, the fact that Dr. Schleifer’s stock options continue to vest according to their original vesting schedule following a voluntary or involuntary termination (other than in connection with a change of control); (iv) we assumed a 5.6% annual increase in medical premiums, 4% annual increase in dental premiums, and no increase in annual disability or life insurance premiums; (v) we assumed that the medical and dental insurance benefits received in 2020, 2021, and 2022 would be taxable and that Dr. Schleifer would be eligible for a tax gross-up for these benefits under the terms of his employment agreement; (vi) although Dr. Schleifer’s employment agreement provides for restrictive covenants, including a six-month non-compete obligation, no specific value has been ascribed to these covenants solely for purposes of this calculation; and (vii) although certain payments to Dr. Schleifer would be subject to potential delays upon separation of service under Section 409A of the Internal Revenue Code, we did not attempt to determine which, if any, payments would be delayed. |
| 2 | Equal to three times the sum of (a) Dr. Schleifer’s 2019 base salary and (b) the average annual cash incentive paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer’s annual cash incentives for performance in 2016, 2017, and 2018. |
| 3 | Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 36 months. |
| 4 | We maintain $1 million of term life insurance covering Dr. Schleifer payable to his designated beneficiary. |
| 5 | Equal to the aggregate amount of the differences between the exercise prices of Dr. Schleifer’s accelerated stock options and the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 31, 2019, the last business day of 2019, of $375.48. None of Dr. Schleifer’s outstanding PSUs would have been earned had a vesting determination been made on December 31, 2019 in respect of performance for the period from the date of grant to December 31, 2019. |
| 6 | Under Dr. Schleifer’s employment agreement, if payments due in connection with a change of control are subject to excise taxes under Section 280G of the Internal Revenue Code, we will cut back the payments if the excise tax can be eliminated by reducing his cash severance payments and benefits by less than 10%. Otherwise, we will pay him an additional “gross up” amount so that his after-tax benefits are the same as though no excise tax had been applied. We have determined that Dr. Schleifer would not have been subject to excise taxes if he had been terminated on December 31, 2019 as a result of a change of control. |
| 7 | Equal to 1.25 times the sum of (a) Dr. Schleifer’s 2019 base salary and (b) the average annual cash incentive paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer’s year-end cash incentive awards for performance in 2016, 2017, and 2018. |
| 8 | Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 18 months. |
| 9 | Equal to the estimated cost of providing Dr. Schleifer’s dependents medical and dental benefits for 18 months. |
| 10 | As discussed in “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments,” unvested stock options held by any employee (including Dr. Schleifer) become immediately exercisable upon his or her death. In addition, any PSUs held by Dr. Schleifer will remain outstanding upon his death, and any vesting or forfeiture of such PSUs will be determined following the completion of the applicable performance period as it otherwise would have been determined if he remained employed by the Company for the duration of such performance period. |
| 11 | Represents 35% of Dr. Schleifer’s 2019 salary over a period of 18 months. We have assumed long-term disability coverage exists pursuant to Dr. Schleifer’s employment agreement for the remaining 65% of Dr. Schleifer’s salary. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 91 / 91 |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
| | Cash Severance | | Benefits Continuation | | Death Benefits4 | | Disability Benefits | | Value of Accelerated Stock Options | | Cutback/ Gross- up6 | | Total Amount | |||||||||
Involuntary Termination Following a Change of Control1 | $ | 10,112,400 | 2 | $ | 199,311 | 3 | | — | | — | $ | 79,965,946 | 5 | | — | $ | 90,277,657 | |||||
Involuntary Termination | $ | 4,213,500 | 7 | $ | 91,742 | 8 | | — | | — | | — | | — | $ | 4,305,242 | ||||||
Death | | — | $ | 89,260 | 8 | | — | | — | | — | 9 | | — | $ | 89,260 | ||||||
Disability | | — | $ | 91,742 | 8 | | — | $ | 630,000 | 10 | | — | | — | $ | 721,742 | ||||||
| | | | | | | | | | | | | | | | | | | | | | | |
1For purposes of these calculations, (i) we used Dr. Schleifer's 2015 base salary and the annual bonuses paid to Dr. Schleifer for performance in 2012, 2013, and 2014, respectively; (ii) we assumed that Dr. Schleifer received his annual bonus that was earned in 2015 and paid in 2016 (described in the Summary Compensation Table on page 61); (iii) we took into consideration, for purposes of determining whether Dr. Schleifer was entitled to receive a gross-up payment under the terms of his employment agreement, the fact that Dr. Schleifer's stock options continue to vest according to their original vesting schedule following a voluntary or involuntary termination (other than in connection with a change of control); (iv) we assumed an 8% annual increase in medical premiums, 5% annual increase in dental premiums, and an increase in annual life insurance premiums from $1,655 to $7,000 in October 2017; (v) we assumed that the medical and dental insurance benefits received in 2016, 2017, and 2018 would be taxable and that Dr. Schleifer would be eligible for a tax gross-up for these benefits under the terms of his employment agreement; (vi) although Dr. Schleifer's employment agreement provides for restrictive covenants, including a six-month non-compete obligation, no specific value has been ascribed to these covenants solely for purposes of this calculation; and (vii) although certain payments to Dr. Schleifer would be subject to potential delays upon separation of service under Section 409A of the Internal Revenue Code, we did not attempt to determine which, if any, payments would be delayed.
2Equal to three times the sum of (a) Dr. Schleifer's 2015 base salary and (b) the average annual bonus paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer's annual bonuses for performance in 2012, 2013, and 2014.
3Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for thirty-six months.
4We maintain $1 million of term life insurance covering Dr. Schleifer payable to his designated beneficiary.
5Equal to the aggregate amount of the differences between the exercise prices of Dr. Schleifer's accelerated stock options and the closing sales price per share of the Company's common stock on the NASDAQ Global Select Market on December 31, 2015 of $542.87.
6Under Dr. Schleifer's employment agreement, if payments due in connection with a change of control are subject to excise taxes under Section 280G of the Internal Revenue Code, we will cut back the payments if the excise tax can be eliminated by reducing his cash severance payments and benefits by less than ten percent. Otherwise, we will pay him an additional "gross up" amount so that his after tax benefits are the same as though no excise tax had been applied. We have determined that Dr. Schleifer would not be subject to excise taxes if he had been terminated on December 31, 2015 as a result of a change of control.
7Equal to 1.25 times the sum of (a) Dr. Schleifer's 2015 base salary and (b) the average annual bonus paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer's annual bonuses for performance in 2012, 2013, and 2014.
8Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for eighteen months.
9As discussed under "Section 4 – Elements of Executive Compensation – Potential Severance Benefits" on page 59, unvested stock options held by any employee (including Dr. Schleifer) become immediately exercisable upon his or her death.
10Represents 35% of Dr. Schleifer's 2015 salary over a period of eighteen months. We have assumed long-term disability coverage exists pursuant to Dr. Schleifer's employment agreement for the remaining 65% of Dr. Schleifer's salary.
67
Executive Compensation
Change in Control Severance Plan
Each of the Named Officers,NEOs, other than our Chief Executive Officer,CEO, participates in our change in control severance plan that was adopted by the board of directors on January 20, 2006. The purposes of the plan are (i) to help us retain key employees, (ii) to help maintain the focus of such employees on our business and to mitigate the distractions caused by the possibility that we may be the target of an acquisition, and (iii) to provide certain benefits to such employees in the event their employment is terminated (or constructively terminated) after, or in contemplation of, a change in control. On November 14, 2008, the change in control severance plan was amended and restated to bring it into compliance with Section 409A of the Internal Revenue Code.
Under the plan, each participant is entitled to receive a cash severance payment in an amount equal to one, or, in designated cases, including with respect to the Named OfficersNEOs other than Dr. Schleifer, two times the sum of the participant'sparticipant’s annual base salary and his or her average annual bonuscash incentive over the prior three years if, within two years after or 180 days before a change in control, either the participant resigns his or her employment for Good Reason (as defined in the plan) or the participant'sparticipant’s employment is terminated by the Company for any reason other than Cause (as defined in the plan). This amount will be paid in a lump sumlump-sum severance payment. A participant so terminated is also entitled to receive a pro rata bonuspro-rata cash incentive for the year in which he or she is terminated based on the portion of the year the participant was employed by us. In addition, for either one or two years, as the case may be, plan participants will receive continuation of health care coverage and welfare benefits provided by us, to the extent permitted by our benefit plans, at a cost no greater than what the participant'sparticipant’s cost would have been if he or she had continued his or her employment with the Company.
In the event that a plan participant resigns his or her employment for Good Reason (which generally conforms to the definition in Section 409A), or the participant'sparticipant’s employment is terminated by the Company for any reason other than Cause, in either case within two years after or 180 days before a change in control, then the participant'sparticipant’s stock options and other equity awards granted under our long-term incentive plans that would have vested prior to or upon the change in control will become vested on the change in control date, and the exercise period of such equity awards, and other equity awards held by the participant that otherwise would have expired, will be extended to the later of (i) thirty30 days following the first date after a change in control in which the shares underlying the equity award may be traded, and (ii) the permitted exercise date in the plan or grant assuming the change in control happened immediately prior to the participant'sparticipant’s termination. However, in no event will any stock option or other equity award be extended (i) beyond the expiration date of the grant, or (ii) such that the grant will be subject to the additional tax under Section 409A of the Internal Revenue Code.
In the event that a participant would become subject to a "golden parachute"“golden parachute” excise tax under Section 4999 of the Internal Revenue Code as a result of severance benefits and payments, the severance benefits and payments owed to the participant shall be reduced to an amount one dollar less than the amount that would subject the participant to the excise tax, unless the total severance benefits/payments net of the excise taxes are greater than the amount that the participant would receive following any such reduction.
68
Executive Compensation
The following table shows the potential payments to our Named OfficersNEOs (other than our Chief Executive Officer)CEO), upon their hypothetical termination (other than for Cause) or resignation for Good Reason, in the two years following, or the six months prior to, a change in control. The information in the
table below is based onassumes an effective termination or resignation date of December 31, 20152019 and is further based on the assumptions set forth in the footnotes to the table; actual values and amounts may differ from those presented below.
92 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
Potential Payments under Change in Control Severance Plan
| | Cash Severance1 | | Benefits Continuation2 | | Value of Accelerated Stock Options/Restricted Stock3 | | Cutback4 | | Total Amount5 | |||||||
George D. Yancopoulos, M.D., Ph.D. | $ | 5,730,360 | $ | 114,488 | $ | 339,406,080 | | — | $ | 345,250,928 | ||||||
Robert E. Landry | $ | 1,440,000 | $ | 111,338 | $ | 17,173,005 | | — | $ | 18,724,343 | ||||||
Neil Stahl, Ph.D. | $ | 1,961,570 | $ | 53,425 | $ | 31,844,172 | | — | $ | 33,859,167 | ||||||
Robert J. Terifay | $ | 1,833,191 | $ | 53,416 | $ | 17,927,425 | | — | $ | 19,814,032 | ||||||
| | | | | | | | | | | | | | | | | |
1Equal to two times the sum of (a) the Named Officer's 2015 base salary and (b) the average annual bonus paid to the Named Officer (other than Mr. Landry)Value of Accelerated Stock Cash Benefits Options/RSAs/ Total Severance1 Continuation2 PSUs3 Cutback4 Amount5 George D. Yancopoulos, M.D., Ph.D. $ 6,680,228 $127,111 $245,460 — $7,052,799 Robert E. Landry $ 2,461,760 $136,930 $7,630,525 — $ 10,229,215 Daniel P. Van Plew $ 2,448,720 $127,680 $2,937,025 — $5,513,425 Andrew J. Murphy, Ph.D. $ 1,887,463 $126,843 $10,446,625 — $ 12,460,931 1 Equal to two times the sum of (a) the NEO’s 2019 base salary and (b) the average annual cash incentives paid to the NEO over the prior three years. 2 Equal to the estimated cost of providing each NEO and his dependents medical, dental, vision, disability, and life insurance coverage for 24 months, plus the estimated cost of providing each NEO tax and financial planning advisory services for 24 months. 3 For stock options, equal to the aggregate amount of the differences between the exercise prices of each NEO’s accelerated “in-the-money” stock options and the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 31, 2019 of $375.48. In the case of Messrs. Landry and Van Plew and Dr. Murphy, the amounts also include the value as of December 31, 2019 of accelerated RSAs. None of Dr. Yancopoulos’s outstanding PSUs would have been earned had a vesting determination been made on December 31, 2019 in respect of performance for the period from the date of grant to December 31, 2019. 4 We have determined (using the assumptions outlined in footnote 5) that all of the NEOs listed in the table above would have been under their applicable “golden parachute” safe harbor limits and not subject to any cutbacks or excise taxes if terminated on December 31, 2019. 5 For purposes of these calculations, (i) we used base salaries as of December 31, 2019 and annual cash incentives paid to the NEOs for performance in 2016, 2017, and 2018, respectively; (ii) we assumed that each NEO received his annual cash incentive that was earned in 2019 and paid in 2020 (described in the Summary Compensation Table above); (iii) we took into consideration, for purposes of determining whether each NEO was subject to a reduction under the terms of the change in control severance plan, the fact that each NEO’s stock options vest in full following an involuntary termination without Cause or termination for Good Reason following a change in control (parachute payments for time vesting stock options and restricted stock were valued using Internal Revenue Code Treas. Reg. Section 1.28G-1 Q&A 24(c)); (iv) we assumed a 5.6% annual increase in medical premiums, 4% annual increase in dental and vision premiums, and no increase in disability or life insurance premiums or employer cost of tax and financial planning advisory services for 2020 and 2021; (v) we assumed that the medical insurance benefits received in 2020 and 2021 would be taxable and that the NEOs would be eligible for a tax gross-up for these benefits under the terms of the change in control severance plan; (vi) although the change in control severance plan provides for restrictive covenants, including a one-year covenant prohibiting the solicitation of company employees, no specific value has been ascribed to these covenants; and (vii) although certain payments to the NEOs would be subject to potential delays upon separation of service under Section 409A of the Internal Revenue Code, we did not attempt to determine which, if any, payments would be delayed.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 93 / 93 |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
ADDITIONAL COMPENSATION INFORMATION
In 2016, we adopted our Cash Incentive Bonus Plan for purposes of allowing our annual cash incentives to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code and permitting us to deduct cash incentive compensation that might otherwise not be deductible by reason of Section 162(m) (as then in effect). Although the Tax Cuts and Jobs Act eliminated the performance-based compensation exception for compensation paid in 2018 and beyond (as discussed under “Our Compensation Processes—Tax Implications” above), we have continued to use the Cash Incentive Bonus Plan for annual cash incentives for the reasons discussed under “Compensation Discussion and Analysis—Our Compensation Philosophy and Objectives” above. For 2019 annual cash incentives for the NEOs, we set up a cash incentive pool and a R&D-related performance goal consisting of (i) the submission of one or more Investigational New Drug Applications, Biologics License Applications, or supplemental Biologics License Applications with the FDA (or its equivalent outside the United States) or (ii) the approval of any regulatory filing of the type described in clause (i) by the FDA or the applicable regulatory authority outside the United States. The Compensation Committee determined that Regeneron’s performance in 2019 exceeded the established goal, thus enabling the funding of the cash incentive pool. The Compensation Committee then exercised “negative discretion” (as permitted under the Plan) to reduce the annual cash incentive amounts otherwise payable to the NEOs according to the cash incentive pool formula. This negative discretion was exercised based on the Compensation Committee’s analysis of all of the relevant facts and circumstances, including the NEOs respective target cash incentive amounts and individual performance in 2019.
The targets for the 2019 annual cash incentives for the NEOs were set as percentages of their respective base salaries as follows: Dr. Schleifer—120%; Dr. Yancopoulos—120%; Mr. Landry—65%; Mr. Van Plew—65%; and Dr. Murphy—65%.
For 2019, Dr. Schleifer’s target cash compensation was set approximately at the median of the Peer Group. In determining the cash incentive target for Dr. Yancopoulos, the Compensation Committee took into consideration the importance of his scientific leadership as President & CSO and the significant contributions he has made to the success of the Company and, specifically, to the discovery and development of the Company’s commercial products, its pipeline of internally developed product candidates, and its platform technologies. The Compensation Committee determined that there were no meaningful comparative data for Dr. Yancopoulos relating to similarly situated executives and that his base salary and cash incentive for 2019 would be set at 85% of Dr. Schleifer’s. In determining the cash incentive targets for 2019 for Mr. Landry, Mr. Van Plew, and Dr. Murphy, the Compensation Committee took into consideration the compensation of similarly situated executive officers at companies in the Peer Group.
The cash incentives were determined through the use of both an individual and a Company performance component with a possible range of 0 to 1.5 for the personal performance multiplier and a possible range of 0 to 2.0 for the Company performance multiplier, depending upon performance during the year. Both the personal performance multiplier and the Company performance multiplier were determined by the Compensation Committee for each NEO based on the Committee’s assessment of the Company’s performance relative to the general corporate goals described below and, in the case of each of Mr. Landry, Mr. Van Plew, and Dr. Murphy, the NEO’s personal performance during the year.
The pipeline-related and other factors that contributed most to the Compensation Committee’s determination of the Company performance multiplier (which was set at 1.85 for 2019) are summarized in the subsection “Compensation Discussion and Analysis—Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives.”
With respect to 2019, the Compensation Committee approved a personal performance multiplier of 1.5 for each of Mr. Landry, Mr. Van Plew, and Dr. Murphy. The personal performance component accounted for 40% of these NEOs’ cash incentives. The Company component was based on a Company performance multiplier that was determined based on the Company’s overall corporate performance (as described above) against 2019 goals that were approved by the board of directors in January 2019. This Company performance component accounted for 60% of the cash
94 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
incentives awarded to Mr. Landry, Mr. Van Plew, and Dr. Murphy. In the case of Drs. Schleifer and Yancopoulos, the Compensation Committee focused exclusively on overall Company performance in 2019 (as described above) when determining their cash incentives and did not utilize a personal performance multiplier.
In determining the personal performance multiplier for Mr. Landry, the Compensation Committee gave special consideration to Mr. Landry’s leadership of and accomplishments in the Company’s accounting, finance, and tax functions and across his other responsibilities, including his leadership of the information technology and real estate & facilities management organizations. In the case of Mr. Landry (who joinedVan Plew, the Compensation Committee focused primarily on Mr. Van Plew’s leadership of and accomplishments in the Company’s Industrial Operations and Product Supply organization, including with respect to expanded manufacturing capacities, preparations for new product launches, successful technology transfer of commercial manufacturing of certain Company products between Regeneron’s Rensselaer, New York and Raheen, Ireland facilities, and successful completion of all regulatory audits concerning the Company’s manufacturing practices in September 2013),2019. In the case of Dr. Murphy, the Compensation Committee considered the progress and continued expansion of the Company’s preclinical and clinical development pipeline, as summarized in the subsection “Compensation Discussion and Analysis—Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives.”
PERQUISITES AND PERSONAL BENEFITS
All employees who participate in our 401(k) Savings Plan, including the NEOs, are eligible to receive certain matching contributions. In each plan year, we averaged his 2013 and 2014 annual bonus amountscontribute to each participant’s account a matching contribution (in the form of $0 and $405,000, respectively, and took into accountshares of our common stock) equal to 50% of a specified percentage of the participant’s compensation that he did not receive a bonus in respect of his performance in 2013.
2Equalthe participant has contributed to theestimated costplan (which was 8% with respect to 2017 and 2018 and 10% with respect to 2019 and 2020), up to a maximum level established under the Internal Revenue Code. Each ofproviding each Named Officerour NEOs participated in our 401(k) Savings Plan during 2019 andhis dependents medical, dental, vision, disability, and life insurance coverage for twenty-four months, plus the estimated cost of providing each Named Officer tax and financial planning advisory services for twenty-four months.
3For stock options, equal toreceived matching contributions in the aggregate amount of $12,500 ($9,500 in thedifferences betweencase of Mr. Van Plew) in theexercise pricesform of shares of our common stock. The contributions were paid quarterly (with the contribution in respect of the fourth quarter of 2019 paid in February 2020) and are included in the compensation amounts reported for eachNamed Officer's accelerated "in-the-money"of our NEOs in the Summary Compensation Table included in this proxy statement. As with all employees, the number of shares of common stockoptions andthat each NEO received on a quarterly basis was determined using theclosing salesaverage market price per share ofthe Company'sour common stock during the applicable quarter.To achieve increased efficiencies and a more secure traveling environment, the Company provides air transportation for certain executive and director travel in accordance with guidelines approved by our board of directors. Based on the
NASDAQ Global Select Marketrecommendation of an independent, third-party security study, the guidelines and our security policy require Drs. Schleifer and Yancopoulos (as well as their spouses and children when they accompany them) to use, as much as practicable, Company-provided aircraft for all business and personal air travel. Regeneron covers the cost of any such personal air travel for up to $250,000 in incremental cost (as described below) annually for each of Drs. Schleifer and Yancopoulos. Family members or other guests may accompany our NEOs and directors during Company-provided air business travel, space permitting, so long as they cover any incremental cost related to such guests (other than with respect to the family members of Drs. Schleifer and Yancopoulos as described above). In addition, in limited circumstances personal use of Company-provided air travel by our other NEOs or directors may be permitted if authorized by the Chairman and any incremental cost is paid by the lead passenger. Any required reimbursement or other payment of the incremental cost is made to the extent permitted by applicable Federal Aviation Administration rules.We determine the incremental cost of any Company-provided personal or guest air travel based on
December 31, 2015the direct variable operating cost. Items included in the calculation include (as applicable) fuel costs; landing, non-home-base hangar or aircraft parking, and ground handling fees; in-flight catering; travel, lodging, and other expenses for flight crew; and other trip-related variable cost, including the use of$542.87.our fractional jet interests. Because Company-provided air transportation is used primarily for business travel, incremental costs exclude fixed costs that generally do not change based on usage, such as (as applicable) flight crew salaries; aircraft purchase or lease costs; depreciation; insurance costs; certain maintenance fees based on minimum usage; and home-base hangar costs. When the aircraft is already flying to a destination for business purposes, only the direct variable costs associated with the guest (for example, catering), if any, are included in determining the aggregate incremental cost to Regeneron. If any aircraft flies empty before picking up or after dropping off a passenger for personal reasons, this “deadhead” segment would be included in the aggregate incremental cost based on the methodology described above. The amount of disallowed corporate tax deductions attributable to Company-provided personal and guest air travel is not included in the NEO incremental cost calculation.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 95 / 95 |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
The security policy also covers secure car transportation and on-site residential security at the primary residence for each of Drs. Schleifer and Yancopoulos. We calculate the incremental costs of the secure car transportation and on-site residential security services provided by Regeneron as the average fuel cost per mile times total miles traveled in connection with such services (if applicable) plus the contractor rate (if any) and salary, taxes, and benefits of drivers attributed to such services based on the number of personal hours expended for such services. Fixed lease costs and routine maintenance that would have been incurred in any event are not included.
Amounts associated with personal or guest Company-provided air and secure car transportation/security services are imputed as income to the NEOs to the extent required by applicable tax regulations. The NEOs do not receive a tax gross-up from us to cover their personal income tax obligations in respect of any such imputed income.
The amounts also disclosed in the “All other compensation” column of the Summary Compensation Table relating to personal and guest use of Company-provided air and secure car transportation/security services in accordance with our security policy attributable to Drs. Schleifer and Yancopoulos are based on the incremental cost resulting from such transportation/services as described above.
The Corporate Governance and Compliance Committee monitors business and any personal or guest Company-provided air travel on a periodic basis.
Additional information regarding perquisites and other personal benefits provided to our NEOs in, or with respect to, 2019 is given in the applicable footnotes to the Summary Compensation Table included in this proxy statement.
Except for Dr. Vagelos, outstanding stock option award agreements for all employees, as well as outstanding RSA and PSU award agreements,11include a “double trigger” provision for the acceleration of vesting of unvested stock options, RSAs, or PSUs upon a termination by the Company without cause or by the employee for good reason within two years following a change in control. Dr. Vagelos’s stock option and PSU awards contain change-of-control provisions consistent with those applicable to the non-employee director equity awards, as described under “Corporate Governance – Compensation of Directors” above.
Our CEO has an employment agreement that provides for certain severance benefits following termination, including following death or disability, resignation following defined “good reason” events, or termination in connection with a change in control. The other NEOs are covered by a change in control severance plan, which provides certain benefits to them and other designated officers if they are terminated in connection with a change in control. In addition, in the case of our CSO, stock option, RSA, and PSU award agreements applicable to his awards granted since December 2015 provide that he would have a “good reason” for terminating his employment with Regeneron upon or within two years after the occurrence of a change in control if the employment of our CEO has ended due to our CEO’s involuntary termination (as defined in the CEO’s employment agreement). Information regarding applicable payments under this employment agreement and change in control severance plan is provided in the subsection “2019 Compensation Tables—Post-Employment Compensation.”
Our NEOs will forfeit any unvested time-based stock options or RSAs upon the termination of their employment for any reason (including disability or retirement) other than death, except as provided in our employment agreement with our CEO, in our change in control severance plan, and in certain stock option agreements with our CSO. In the event of the death of an employee, any unvested stock options held by such employee become immediately exercisable, and any shares subject to RSAs/RSUs will become fully vested. In the case of PSU awards to our CEO and CSO (as well as Dr. Vagelos, as described under “Corporate Governance – Compensation of Directors” above), upon a termination of employment due to death (or retirement when “retirement eligible,” as discussed below), the PSU award will remain outstanding, and any vesting of the PSUs will be determined following the completion of the applicable performance period. For information regarding the value of accelerated stock options, RSAs, and PSUs held by our CEO and other NEOs (as applicable) as of December 31, 20152019, see the subsection “2019 Compensation Tables—Post-Employment Compensation” under “Value of Accelerated Stock Options/PSUs” in the table entitled “Potential Severance Payments
| 11 | In the case of PSUs, if any PSUs vest upon a change of control (as a result of performance exceeding the relevant TSR goal for the period from the date of grant to the date of the change of control), the shares earned will be delivered upon the earlier of (a) a termination by the Company without cause or by the employee for good reason within two years following the change in control and (b) the fifth year anniversary of the date of grant. |
96 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
COMPENSATION-RELATED MATTERS/COMPENSATION DASHBOARD
under Dr. Schleifer’s Employment Agreement” (for our CEO) and under “Value of Accelerated Stock Options/RSAs/ PSUs” in the table entitled “Potential Payments under Change in Control Severance Plan” (for other NEOs).
When employees (other than our CEO and, with respect to stock option awards commencing in December 2018 and PSU awards, our CSO and Dr. Vagelos) retire, they forfeit all unvested restricted stock.
4- time-based stock options, RSAs, and PSUs. An employee considered “retirement eligible” upon separation under our employee policies as in effect from time to time has the remaining life of the 10-year stock option term to exercise stock options that are vested as of the date of his or her retirement. In addition, the award agreements with our CSO and Dr. Vagelos relating to their 2018 and 2019 stock option awards and 2019 PSU awards provide that their respective awards will continue to vest in accordance with the terms of the applicable award agreement so long as they are “retirement eligible” upon separation.
The severance benefits provided to our NEOs are designed to promote stability and continuity of our senior management and are intended to preserve employee morale and productivity and encourage retention in the face of the disruptive impact of an actual, threatened, or rumored change in control of the Company. The severance
plan,benefits were established following a review of comparable practices at the Company’s peer companies and with the advice of the Compensation Committee’s consultant.We have no pension, deferred compensation, or retirement plans applicable to our NEOs, other than our 401(k) Savings Plan described above.
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of Regulation S-K, we are required to disclose the median of the annual total compensation of our employees (excluding our principal executive officer), the annual total compensation of our principal executive officer, Dr. Schleifer, and the ratio of these two amounts.
We have determined the total
amount for Mr. Landry has not been "cut back"compensation of our median employee (based on the 2019 annual total compensation of our employees, excluding Dr. Schleifer) to be $139,055. The total 2019 compensation of Dr. Schleifer, ashe would be in a more favorable net after-tax position without any such reduction. None of the other Named Officers listed in the table above would have been expected to receive severance payments in excess of his applicable "golden parachute" safe harbor amount.
5For purposes of these calculations, (i) we used base salaries as of December 31, 2015 and annual bonuses paid to the Named Officers (other than Mr. Landry) for performance in 2012, 2013, and 2014, respectively; in the case of Mr. Landry (who joined the Company in September 2013), we averaged his 2013 and 2014 annual bonus amounts of $0 and $405,000, respectively, and took into account that he did not receive a bonus in respect of his performance in 2013; (ii) we assumed that each Named Officer received his annual bonus that was earned in 2015 and paid in 2016 (describedreported in the Summary Compensation Table above, was $21,455,117. Accordingly, the ratio of the 2019 annual total compensation of Dr. Schleifer to the median of the 2019 annual total compensation of our employees was approximately 154 to 1.Consistent with our process for identifying the median employee for 2017 and 2018, for 2019 we identified the median employee as of December 31, 2019 by (i) aggregating for each applicable employee (a) annual base salary for salaried employees (or wages plus overtime, based on
page 61); (iii) we took into consideration,annual work schedule, for permanent hourly employees), (b) the annual cash incentive, and (c) the grant date fair value of any equity awards granted during 2019, and (ii) ranking this compensation measure from lowest to highest. This calculation was performed for all employees, excluding Dr. Schleifer, whether employed on a full-time, part-time, or seasonal basis. For purposes ofdetermining whether each Named Officer was subjectidentifying the median employee, we converted amounts paid in foreign currencies to U.S. dollars based on the applicable 2019 average exchange rate.We believe that the pay ratio reported above is a
reduction underreasonable estimate calculated in a manner consistent with SEC rules based on our internal records and thetermsmethodology described above. Because the SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates, and assumptions in calculating their own pay ratios.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 97 / 97 |

APPROVAL OF SECOND
AMENDMENT AND
RESTATEMENT OF LONG-
TERM INCENTIVE PLAN
The Board of Directors Unanimously Recommends a Vote FOR Approval of the change in control severance plan, the fact that each Named Officer's stock options vest following an involuntary termination without Cause or termination for Good Reason following a change in control (parachute payments for time vesting stock optionsSecond Amended and restricted stock were valued using Internal Revenue Code Treas. Reg. Section 1.28G-1 Q&A 24(c)); (iv) we assumed an 8% annual increase in medical premiums, 5% annual increase in dental premiums, 4% increase in vision premiums, and no increase in disability or life insurance premiums or employer cost of tax and financial planning advisory services for 2016 and 2017; (v) we assumed that the medical insurance benefits received in 2016 and 2017 would be taxable and that the Named Officers would be eligible for a tax gross-up for these benefits under the terms of the change in control severance plan; (vi) although the change in control severance plan provides for restrictive covenants, including a one-year covenant prohibiting the solicitation of company employees, no specific value has been ascribed to these covenants; and (vii) although certain payments to the Named Officers would be subject to potential delays upon separation of service under Section 409A of the Internal Revenue Code, we did not attempt to determine which, if any, payments would be delayed.
69
Executive Compensation
Corporate Governance Aspects of theRestated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive PlanPlan.
98 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |

PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN
INTRODUCTION
It would be difficult to overstate the importance of this proposal to the Company and its core strategy. Using equity awards to motivate, reward, and retain all of our employees has been a key part of Regeneron’s strategy and compensation philosophy since the Company’s inception. We firmly believe that this compensation model, including the use of equity awards as a primary long-term employee incentive, directly supports Regeneron’s core strategy to create and advance a high-quality, internally developed product pipeline and has helped us establish a culture where employees focus on our mission and drug discovery and development. A primary underpinning of our pay philosophy is to award equity-based pay toall employees, not just senior executives, to ensure that when we deliver for patients and for shareholders, everyone shares in the potential upside growth. In each of the last five years, approximately 90% of the employee equity grants (both based on grant date fair value and number of underlying shares) were awarded to our employees other than our NEOs12. We believe that our broad-based equity program, which ensures that all employees can share in our future potential in the same way as executives, represents one of our competitive advantages.
| Key Takeaways | |
| 1 | Our equity compensation program supports all of our employees, not just our NEOs, with approximately 90% of recent annual equity grants awarded to employees other than our NEOs. |
| 2 | We granted PSUs to our CEO and CSO as a component of their 2019 annual equity awards. This and other carefully calibrated changes to our compensation program were based on investor feedback. |
| 3 | Our 2019 burn rate was at the lowest level in the last seven years due to specific steps we took to manage dilution from equity compensation. |
| 4 | We are asking our shareholders to approve the same number of shares for equity grants that we requested previously and are committed to ensuring that shares available under our long-term incentive plan will be sufficient for at least three years. |
| 5 | Our compensation model underpins our strategy of creating and advancing a high-quality, internally developed product pipeline, which delivered six important medicines and eight additional key indications for these products in the past decade. |
A breakdown of employee equity grants in 2019 (when approximately 92% of such grants were awarded to non-NEOs) is provided below.
2019 Employee Equity Grants

We will not be able to execute our strategy without shareholder support of our equity compensation plans and this proposal. We are making this request after a rigorous assessment of our strategy and goals and after taking affirmative steps to manage our burn rate and dilution without compromising our all-employee equity compensation model. These steps include a significant reduction in the number of shares underlying the annual equity awards of our eligible employees, including our CEO, by nearly 60% between 2012 and 2019; and the addition of full-value awards (RSAs and RSUs) to the annual long-term incentive mix for our employees and thereby reducing the number of shares required for these awards. The results of these efforts are illustrated by the chart below.13
| 12 | See the list of our “Named Executive Officers” or “NEOs” in the “Compensation Discussion and Analysis” section of this proxy statement. |
| 13 | Further information regarding our burn rate is provided in the subsection “Key Equity Metrics” below. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 99 / 99 |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
Regeneron Stock Utilization vs. Headcount*
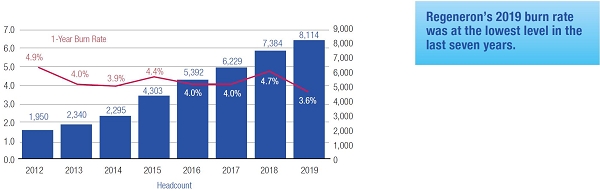
| * | Burn rate calculated by dividing the number of shares subject to equity awards (stock options, RSAs, and RSUs) granted during the year by the basic weighted-average number of shares of common stock and Class A stock outstanding during the year. PSUs are to be reflected in the burn rate calculation for the year in which they are earned and, therefore, do not impact the burn rate shown in the chart. Headcount numbers based on the number of employees as of December 31 of the applicable year. |
In the last decade, we sought shareholder approval of additional shares for our long-term incentive plans three times: 2011, 2014, and 2017. Each time, we requested the same number of shares – 12 million – and lived up to our commitment not to seek additional shares earlier than three years following approval. We have been able to keep the same size of share requests while maintaining our all-employee equity compensation model and increasing the number of our employees to support our growth (by almost 700% in the last 10 years) because we took the steps to manage our burn rate and dilution discussed above. Once again, we are asking shareholders to approve 12 million additional shares for equity grants and are committed to ensuring that these additional shares (together with the shares currently available under the Current Plan or that may otherwise become available under the New Plan (each as defined below)) will satisfy the Company’s compensation and recruiting needs for no less than three years.
Our pay philosophy and equity compensation strategy are discussed further in the CD&A section of this proxy statement.
We appreciate your support of this important proposal.
SUMMARY OF PROPOSAL AND KEY CHANGES
We currently use one equity incentive plan—the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (referred to in this subsection as the "Plan"(the “Current Plan”) is the only plan currently used by the Company —to grant equity awards. It has beenawards to our directors, executive officers, and other employees. The Current Plan was approved by our shareholders in 2017; it amended and restated the equity incentive plan that was approved by our shareholders in 2014. On April 3, 2020, the board of directors, upon the recommendation of the Compensation Committee (made following the Committee’s consultation with its independent compensation consultant), and subject to shareholder approval, adopted the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan in the form attached as Appendix B to this proxy statement, which amends and restates the Current Plan (the “New Plan”). Among other things, the New Plan:
| • | increases the number of shares of our common stock available for issuance thereunder by 12 million; |
| • | extends the period during which awards can be granted to 2030; and |
| • | incorporates new annual limits on the equity awards to our non-employee directors and the current Chairman of the Board as required by the Settlement (as defined and described below). |
100 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
CORPORATE GOVERNANCE ASPECTS OF THE SECOND AMENDED AND RESTATED REGENERON PHARMACEUTICALS, INC. 2014 LONG-TERM INCENTIVE PLAN
Similar to the Current Plan, the New Plan was designed to include a number of provisions that promote best practices by reinforcing the alignment
between equity compensation arrangements for eligible employees and non-employee directors onand the one hand, and shareholders' interests on the other hand. Theseof shareholders. The provisions that promote such best practices include:
Provision | Description | |
| No Discounted StockOptions or StockAppreciation Rights | Stock options and stock appreciation rights are not granted with an exercise or base price less than the fair market value of common stock (as defined in the New Plan) on the date of grant. | |
| No Stock Option orStock Appreciation RightRe-pricing or Exchange | Except for equitable adjustments in connection with specific corporate transactions (such as stock splits, recapitalizations, reorganizations, mergers, consolidations, and similar transactions), the New Plan does not permit a decrease in the exercise price or base price of a stock option or stock appreciation right granted under the New Plan through settlement, cancellation, forfeiture, exchange, surrender, or otherwise below the fair market value of common stock (as defined in the New Plan) on the date of grant. | |
| Recoupment (Clawback)Policy |
| |
| Awards granted to our officers and other | ||
| Independent Administration | The New Plan is administered by the Compensation Committee, which is intended to be comprised solely of non-employee directors each of whom meets the additional independence criteria applicable to compensation committee members under the listing standards of The NASDAQ Stock Market LLC and qualifies as a | |
| No | The New Plan does not contain an | |
| No Tax Gross-ups | The New Plan does not provide for any tax gross-ups. |
KEY EQUITY METRICS
The following table summarizes some key metrics relating to the equity component of our compensation program. When evaluating the information below, it is important to keep in mind the 50% increase in the number of our employees from 2017 to 2019 and our all-employee equity award strategy encompassing initial equity grants to all new hires as well as a comprehensive annual equity program covering all levels of employees:
| 2019 | 2018 | 2017 | |
| Unadjusted Burn Rate1 | 3.58% | 4.68% | 4.04% |
| Adjusted Burn Rate1 | 4.46% | 5.20% | 4.13% |
| Overhang2 | 26.47% | 27.35% | 28.13% |
| Dilution3 | 21.42% | 20.94% | 19.66% |
| 1 | Calculated by dividing (a) the sum of the number of shares subject to (i) stock options, RSAs, and RSUs granted during the year and (ii) PSUs earned during the year (if any), by (b) the basic weighted-average number of shares of common stock and Class A stock outstanding during the year. For “Adjusted Burn Rate,” a multiplier of 2.5 is applied to RSAs, RSUs, and PSUs. |
| 2 | Calculated by dividing (a) the sum of (i) the number of shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming maximum payouts earned)) outstanding at the end of the year and (ii) the number of shares available for future grants under the Current Plan at the end of the year, by (b) the sum of (i) the number of shares of common stock and Class A stock outstanding at the end of the year, (ii) the shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming maximum payouts earned)) outstanding at the end of the year, and (iii) the number of shares available for future grants under the Current Plan at the end of the year. |
| 3 | Calculated by dividing the number of shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming maximum payouts earned)) outstanding at the end of the year by the sum of (i) the number of shares of common stock and Class A stock outstanding at the end of the year and (ii) the shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming maximum payouts earned)) outstanding at the end of the year. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 101 / 101 |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
70
ExecutiveMATERIAL TERMS OF THE NEW PLAN SUBMITTED FOR SHAREHOLDER APPROVAL
The following description of the material terms of the New Plan is qualified in its entirety by the terms of the plan document, which is attached as Appendix B to this proxy statement.
Available Shares.As of April 14, 2020, the record date for this meeting, there were 10,150,052 shares of common stock remaining available for issuance under the Current Plan. Utilizing management’s projections, which are based on past practices and projected headcount, the Compensation Committee has determined that the 12 million shares newly authorized under New Plan being submitted for shareholder approval (along with the remaining 10,150,052 shares authorized under the Current Plan) should satisfy the Company’s equity compensation needs for at least the next three years. If the New Plan is not approved by shareholders, to achieve our equity compensation objectives we will only be able to utilize the remaining 10,150,052 shares of common stock (as of the record date for this meeting) available under the Current Plan (plus any shares subject to awards which are returned to the plan upon the expiration, forfeiture, surrender, exchange, cancellation, or termination of previously granted awards). We believe that the current share pool would be insufficient to satisfy our expected recruiting and compensation needs. If the New Plan is approved by shareholders, an aggregate of 22,150,052 shares will be available for grants under the New Plan. Shares of common stock underlying or issued pursuant to equity awards previously granted under the Current Plan, the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (the “2014 Plan”), or the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan (the “2000 Plan”) that would otherwise have again become available under the Current Plan, the 2014 Plan, or the 2000 Plan upon the expiration, forfeiture, surrender, exchange, cancellation, or termination of such previously granted awards, in whole or in part, for any reason, including the surrender of shares to pay the exercise price or satisfy withholding tax obligations in connection with the grant, exercise, or vesting of all or part of an award will be added to the pool of shares available for grant under the New Plan. As of the record date for this meeting, there were 26,047,513 shares subject to outstanding awards (stock options, RSAs, RSUs, and PSUs) previously issued under the Current Plan, the 2014 Plan, and the 2000 Plan. All of the shares reserved and available for issuance under the New Plan, the various limits set forth in the New Plan, and the awards made thereunder will generally be subject to equitable adjustment upon the occurrence of any stock dividend or other distribution, recapitalization, stock split, subdivision, reorganization, merger, consolidation, combination, repurchase, or share exchange, or other similar corporate transaction or event. Shares issued pursuant to awards that are assumed by us in corporate transactions will not count against the shares available under the New Plan.
Plan Term.The term of the New Plan runs through April 3, 2030 (although awards granted prior to such date may continue in effect beyond that date in accordance with their respective terms).
Administration.The New Plan is to be administered by the Compensation Committee of the board of directors. Each member of the Compensation Committee is intended to be a “Non-Employee Director” (within the meaning of Rule 16b-3 under the Exchange Act) and to otherwise qualify as independent to the extent required under applicable law, regulations, and listing standards. The New Plan allows the Compensation Committee to delegate to one or more executive officers of the Company the authority to exercise any of the Compensation Committee’s responsibilities under the New Plan, including the authority to grant awards thereunder (subject to certain limitations set forth in the New Plan).
Amendment; Term.The New Plan may be amended by the board of directors, subject to shareholder approval where necessary, to satisfy certain regulatory or legal requirements. The New Plan will terminate no later than April 3, 2030. However, awards granted before such termination will extend beyond that date in accordance with their terms.
Participants.Awards under the New Plan may be made to employees of the Company, including officers of the Company (whether or not they are directors), and to non-employee directors and consultants. Non-employee directors receive grants subject to the limits described under “Non-Employee Director Awards” below. As of the record date for this meeting, we had 8,095 employees and nine non-employee directors, who would have been eligible to receive awards under the New Plan; as of that date, no consultants would have been eligible to receive awards thereunder.
Awards.There are generally five types of awards that may be granted under the New Plan: Stock Options (including both incentive stock options (referred to as “ISOs”) within the meaning of Section 422 of the Internal Revenue Code
102 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
Equityand nonqualified stock options (referred to as “NQSOs”), which are options that do not qualify as ISOs), Restricted Stock, Phantom Stock (including RSUs and PSUs (each as defined below)), Stock Appreciation Rights, and Stock Bonus awards. In addition, the Compensation Committee in its discretion may make other awards valued in whole or in part by reference to, or otherwise based on, shares of common stock. The maximum number of shares which may be made subject to grants of ISOs during the term of the New Plan Informationis 22,150,052. While the New Plan will permit the issuance of ISOs, the Compensation Committee determined to stop using ISOs in November 2012 and has not authorized any ISOs since then.
Vesting.Awards become exercisable or otherwise vest at the times and upon the conditions that the Compensation Committee may determine, as reflected in the applicable award agreement. The Compensation Committee has the authority to accelerate the vesting and/or exercisability of any outstanding award at such times and under such circumstances as it, in its sole discretion, deems appropriate (for instance, upon a “Change in Control” of the Company, as defined in the New Plan).
Stock Options.Options entitle the holder to purchase shares of common stock during a specified period at the purchase price specified by the Compensation Committee, which may not be less than 100% of the fair market value of the common stock on the day the option is granted (determined as (i) the average of the high and low sales price per share of common stock on the securities exchange or system on which such stock is principally traded on such date or, if such date is not a trading day, on the last preceding date on which there was a sale of such stock on such exchange or system, or (ii) if the shares of common stock are not then listed, or the value of such shares is not otherwise determinable, such value as determined by the Compensation Committee in good faith). Each option granted under the New Plan will be exercisable for a maximum period of 10 years from the date of grant (subject to early termination such as upon a termination of employment), or such lesser period as the Compensation Committee shall determine. Options may be exercised, in whole or in part, by the payment in cash of the full option price of the shares purchased, by tendering shares of common stock with a fair market value equal to the option price of the shares purchased, or by other methods in the discretion of the Compensation Committee. The vesting schedule for options to be granted under the New Plan will be determined by the Compensation Committee. Options that are exercisable as of the date of a participant’s termination of service with the Company may be exercised after such date for the period set forth in the option agreement or as otherwise determined by the Compensation Committee. In the event of the death of a participant, any then outstanding and unexercisable options held by such participant shall become exercisable by the participant’s heirs or legal representatives. Options held by a participant upon termination from the Company’s service for cause shall immediately expire (whether or not then exercisable). Except in connection with specific corporate transactions (such as stock splits, recapitalizations, reorganizations, mergers, consolidations, and similar transactions), the New Plan does not permit a decrease in the exercise price of a stock option granted under the New Plan through settlement, cancellation, forfeiture, exchange, surrender, or otherwise below the fair market value of common stock (as defined in the New Plan and described above) on the date of grant.
Restricted Stock.Restricted stock awards (“RSAs”) consist of a grant of shares of restricted common stock. The Compensation Committee may determine the price, if any, to be paid by a participant for each share of restricted stock subject to an RSA. Unless otherwise provided in the applicable award agreement, an RSA holder may vote, and if the participant remains in the service of the Company until the applicable “Vesting Date” as defined in the New Plan, he or she may generally receive all dividends on, all shares subject to the RSA. However, such holder may not transfer such shares until the applicable Vesting Date. If for any reason before the applicable Vesting Date an RSA holder ceases to be in the employment or service of the Company, unless otherwise provided in the applicable award agreement or determined by the Compensation Committee, the holder shall be required to forfeit to the Company any such RSA together with any dividends paid thereon.
Phantom Stock.A phantom stock award is an award of the right to receive common stock or an amount of cash based on the value of the common stock at a future date, subject to such restrictions, if any, as the Compensation Committee may impose at the date of grant or thereafter, which restrictions may lapse separately or in combination at such times, under such circumstances (including without limitation a specified period of employment or the achievement of certain performance goals), in such installments, or otherwise, as the Compensation Committee may determine. Time-based restricted stock units (“RSUs”) and performance-based restricted stock units (“PSUs”) are each a type of phantom stock award permitted under the New Plan. A phantom stock award settled in cash does not reduce the number of shares of common stock with respect to which awards may be granted under the New Plan.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 103 / 103 |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
Stock Appreciation Rights.Stock appreciation rights (“SARs”) entitle the holder to a payment (in cash or shares) equal to the appreciation in the value of our common stock during a specified period above the base price specified by the Compensation Committee, which may not be less than 100% of the fair market value of the common stock (as defined in the New Plan and described above) on the day the SAR is granted. Each SAR granted under the New Plan will be exercisable for a maximum period of 10 years from the date of grant (subject to early termination such as upon a termination of employment), or such lesser period as the Compensation Committee shall determine. The vesting schedule for SARs to be granted under the New Plan will be determined by the Compensation Committee. SARs that are exercisable as of the date of a participant’s termination of service with the Company may be exercised after such date for the period set forth in the SAR agreement or as otherwise determined by the Compensation Committee. Except in connection with specific corporate transactions (such as stock splits, recapitalizations, reorganizations, mergers, consolidations, and similar transactions), the New Plan does not permit a decrease in the base price of a SAR granted under the New Plan through settlement, cancellation, forfeiture, exchange, surrender, or otherwise below the fair market value of common stock on the date of grant.
Stock Bonus.If the Compensation Committee grants a stock bonus award (which an award that is not subject to vesting conditions), a certificate for the shares of common stock consisting of such stock bonus is issued in the name of the participant to whom such grant was made.
Recoupment (Clawback).Awards granted to our officers and other employees under the New Plan will be subject to recoupment or reduction in accordance with the terms of our policy regarding recoupment or reduction of incentive compensation (clawback policy).
Non-Employee Director Awards; Non-Employee Director Compensation Limit.Non-employee directors of the Company are generally eligible to receive awards under the Plan (other than ISOs) under such terms and conditions as the Compensation Committee may determine. During any calendar year, the aggregate number of shares subject to one or more awards granted to a non-employee director in a year may not exceed 12,750 shares, except that during the first calendar year in which a non-employee director serves on the board of directors, such limit will be 34,000 shares;provided, however, that any awards granted to a non-employee director during or prior to 2023 shall be subject to the limitations set forth in the Settlement (as defined and described below). In addition, during any calendar year, the aggregate number of shares subject to one or more awards granted to a non-employee director then serving as chairman of the board of directors in such year may not exceed 25,500 shares, except that during the first calendar year in which a non-employee director serves as chairman of the board of directors, such limit will be 68,000 shares;provided, however, that any awards granted to the current Chairman of the Board, Dr. Vagelos, during or prior to 2022 shall be subject to the limitations set forth in the Settlement. For purposes of applying this limit, an award other than an NQSO or SAR is treated as an award of one and one-half (1.5) shares for each share actually subject to such award, and an award of an NQSO or SAR is treated as an award of one share for each share actually subject to such award.
In December 2018, the Supreme Court of the State of New York, County of New York entered an order and final judgment approving the Stipulation of Compromise and Settlement, dated as of October 5, 2018, by and among the Plaintiffs and the Individual Defendants (each as defined therein) and the Company, as nominal defendant (the “Settlement”) in the shareholder derivative actions entitled Cement Masons Local 780 Pension Fund, et ano. v. Leonard S. Schleifer, et al., Index No. 654453/2015 (N.Y. Co.) (filed December 30, 2015) andPublic Employees’ Retirement System of Mississippi v. Leonard S. Schleifer, et al., Index No. 656813/2017 (N.Y. Co.) (filed November 8, 2017). The terms of the Settlement impose the following limits on equity compensation awarded to the non-employee directors of the Company and the current Chairman of the Board, Dr. Vagelos, respectively:
| 1 | The aggregate annual equity compensation per non-employee director shall be limited to $695,000 through 2020, as valued for purposes of the Company’s financial statements; thereafter, through 2023, the aggregate annual equity compensation per non-employee director may be increased by up to 5% annually; and |
| 2 | The aggregate annual equity compensation for the current Chairman of the Board, Dr. Vagelos, shall be limited to ten times the corresponding annual equity compensation for the non-employee directors through 2022,14 as valued for purposes of the Company’s financial statements. |
| 14 | Equity awards to the current Chairman of the Board are granted in December annually (along with the equity awards to our full employee base), and each such December grant corresponds to the awards granted to the non-employee directors in January of the following year. |
104 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
Notwithstanding the foregoing, during the first calendar year in which a non-employee director serves on the board of directors, in addition to the annual equity award compensation set forth in clause (1) above (which shall be prorated for such calendar year based on the date such director is elected to the board), the Settlement permits a non-employee director to be awarded an inducement equity award of up to 5/3rds of (a) the number of shares of common stock underlying the most recent annual equity grant to non-employee directors or (b) the grant date fair value (as valued for purposes of the Company’s financial statements) of such grants. In addition, the Settlement’s limits on compensation awarded to the Company’s directors (including those described above) shall not apply to any successor to Dr. Vagelos to the position of Chairperson of the Board.15
CERTAIN FEDERAL INCOME TAX CONSEQUENCES
Set forth below is a discussion of certain federal income tax consequences with respect to options that may be granted pursuant to the New Plan. The following discussion is a brief summary only, and reference is made to the Internal Revenue Code and the regulations and interpretations issued thereunder for a complete statement of all relevant federal tax consequences. This summary is given as of the date of this proxy statement, is not intended to be exhaustive, and does not describe state, local, or foreign tax consequences of participation in the New Plan. Recipients of awards under the New Plan are advised to consult with their personal tax advisors with regard to all tax consequences arising with respect to their awards.
Incentive Stock Options.In general, no taxable income is realized by a participant upon the grant of an ISO. If shares of common stock are issued to a participant (“Option Shares”) pursuant to the exercise of an ISO granted under the New Plan and the participant does not dispose of the Option Shares within the two-year period after the date of option grant or within one year after the receipt of such Option Shares by the participant (an earlier disposition being referred to as a “disqualifying disposition”), then generally (i) the participant will not realize ordinary income upon exercise and (ii) upon sale of such Option Shares, any amount realized in excess of the exercise price paid for the Option Shares will be taxed to such participant as capital gain (or loss). The amount by which the fair market value of the common stock on the exercise date of an ISO exceeds the purchase price generally will constitute an item which increases the participant’s “alternative minimum taxable income.” If Option Shares acquired upon the exercise of an ISO are disposed of in a disqualifying disposition, the participant generally would include in ordinary income in the year of disposition an amount equal to the excess of the fair market value of the Option Shares at the time of exercise (or, if less, the amount realized on the disposition of the Option Shares) over the exercise price paid for the Option Shares. Subject to certain exceptions, an ISO generally will not be treated as an ISO if it is exercised more than three months (other than by reason of death) following termination of employment. If an ISO is exercised at a time when it no longer qualifies as an ISO, such option will be treated as an NQSO as discussed below. The Company is not entitled to a federal income tax deduction on the grant or exercise of an ISO or on the participant’s disposition of the Option Shares after satisfying the holding period requirements described above. If the holding periods are not satisfied, the Company will be entitled to a deduction in the year the participant disposes of the Option Shares in an amount equal to the ordinary income recognized by the participant. Any additional gain or loss realized will be taxed as a short-term or long-term capital gain or loss, as the case may be, and may not be deducted by the Company.
Nonqualified Stock Options.In general, no taxable income is realized by a participant upon the grant of an NQSO. Upon exercise of an NQSO, the participant generally would include in ordinary income at the time of exercise an amount equal to the excess, if any, of the fair market value of the Option Shares at the time of exercise over the exercise price paid for the Option Shares. In the event of a subsequent sale of Option Shares received upon the exercise of an NQSO, any appreciation or depreciation of the shares after the date on which taxable income is realized by the participant in respect of the option exercise will be taxed as capital gain in an amount equal to the excess of the sale proceeds for the Option Shares over the participant’s basis in such Option Shares. The participant’s basis in the Option Shares will generally equal the amount paid for the Option Shares plus the amount included in ordinary income by the participant upon exercise of the NQSO. The Company generally will have a deduction in an amount equal to the amount of ordinary income recognized by the participant in the Company’s tax year during which the participant recognizes the ordinary income.
| 15 | The terms of the Settlement and certain related matters are described in detail in the Company’s Current Report on Form 8-K filed with the SEC on October 12, 2018. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 105 / 105 |

| PROPOSAL 03: APPROVAL OF SECOND AMENDMENT AND RESTATEMENT OF LONG-TERM INCENTIVE PLAN |
INTERESTS OF CERTAIN PERSONS IN MATTERS TO BE ACTED UPON
Officers of the Company, including the NEOs, employees, consultants, and non-employee directors of the Company are eligible to receive awards under the New Plan in the discretion of the Compensation Committee. Future grants under the New Plan will be made at the discretion of the Compensation Committee and thus are not determinable at this time.
SEC REGISTRATION
We intend to file with the SEC a registration statement on Form S-8 relating to the registration of additional shares of common stock issuable under the New Plan pursuant to the Securities Act as soon as practicable after approval of the New Plan by our shareholders.
EQUITY COMPENSATION PLAN INFORMATION
The following table shows information with respect to securities authorized for issuance under the equity compensation plans maintained by the Company as of December 31, 2015.2019.
Plan Category | (a) Number of securities to be issued upon exercise of outstanding options, warrants, and rights | | (b) Weighted-average exercise price of outstanding options, warrants, and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||
Equity compensation plans approved by security holders1 | 23,165,769 shares of common stock | $ | 236.75 | 9,711,439 shares of common stock3 | |||
Equity compensation plans not approved by security holders2 | – | $ | – | 44,246 shares of Class A stock | |||
| | | | | | | | |
Total | 23,165,769 shares of common stock | $ | 236.75 | 9,755,685 shares of common stock and Class A stock | |||
| | | | | | | | |
1The equity compensation plans approved by the security holders are the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan and the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan is the only plan currently used by the Company to grant equity awards.
2A B C Plan Category Number of securities to be issued upon
exercise of outstanding options,
warrants, and rightsWeighted-average exercise price
of outstanding options, warrants,
and rightsNumber of securities remaining available for
future issuance under equity compensation plans
(excluding securities reflected in column (a))Equity compensation plans
approved by security holders128,729,3003shares of common stock $337.244 9,546,628 shares of common stock5 Equity compensation plans not
approved by security holders2— $ — 44,246 shares of Class A stock Total 28,729,300 shares of common stock $ 337.24 9,590,874 shares of common stockand Class A stock 1 The equity compensation plans approved by the security holders are the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan; the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan; and the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan is the only plan currently used by the Company to grant equity awards. 2 The equity compensation plan not approved by the security holders is the Executive Stock Purchase Plan. It was adopted in 1989 and provides for the Compensation Committee of the board of directors to award employees, directors, consultants, and other individuals who render service to the Company the right to purchase Class A stock at a price set by the Compensation Committee. The Plan provides for the vesting of shares as determined by the Compensation Committee; should the Company’s relationship with a Plan participant terminate before all shares are vested, unvested shares will be repurchased by the Company at a price per share equal to the original amount paid by the Plan participant. As of December 31, 2019, there were no unvested shares and 44,246 shares of Class A stock available for future grants under the Plan. 3 This amount includes (i) 28,609,277 shares to be issued upon exercise of outstanding options, (ii) 60,627 shares to be issued upon vesting of RSUs, and (iii) 59,396 shares to be issued upon vesting of PSUs (assuming maximum payouts earned) . 4 The calculation of the weighted-average exercise price does not include the 60,627 shares to be issued upon vesting of RSUs or the 59,396 shares to be issued upon vesting of PSUs (assuming maximum payouts earned), as RSUs and PSUs do not have an exercise price. 5 This amount is net of 1,041,763 outstanding RSAs. As these shares are considered issued and outstanding upon grant, they are not included in the amounts reported in column (a). 106 / 
2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING
ADVISORY VOTE ON
COMPENSATION OF NAMED
EXECUTIVE OFFICERS
(SAY ON PAY)
The Board of Directors Unanimously Recommends a Vote, on an Advisory Basis, FOR Approval of the Compensation of Our Named Executive Officers as Disclosed in This Proxy Statement.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 107 / 107 |
| PROPOSAL 04: ADVISORY VOTE ON COMPENSATION OF NAMED EXECUTIVE OFFICERS |
As required by Section 14A of the Exchange Act, we are seeking, on an advisory basis, shareholder approval of the compensation of our NEOs as disclosed above (“say-on-pay” proposal). Specifically, shareholders are being asked to approve the following advisory resolution:
RESOLVED, that the shareholders of Regeneron Pharmaceuticals, Inc. hereby approve the compensation of the Company’s Named Executive Officers, as disclosed in the Company’s proxy statement relating to the Company’s 2020 Annual Meeting (the “Proxy Statement”) pursuant to the compensation disclosure rules of the Securities and Exchange Commission (which disclosure includes the Compensation Discussion and Analysis, the compensation tables, and the related compensation information contained in the Proxy Statement).
In determining to recommend that shareholders approve the say-on-pay proposal, the board of directors to award employees, directors, consultants,considered, among other factors discussed under “Compensation Discussion and other individuals who render service toAnalysis” above, that the Company had achieved its corporate goals in 2019 and each of the right to
- other two years since the most recent say-on-pay vote (2017 and 2018). Information regarding NEO compensation for each of these years is provided in the Summary Compensation Table included in this proxy statement.
purchase Class A stock at a price set by
As an advisory vote, this proposal is non-binding on the Company. However, the board of directors and the Compensation Committee. The Plan provides forCommittee value your opinion and will review and consider the vestingvoting results in connection with their ongoing evaluation of shares as determined by the Compensation Committee; should the Company's relationship with a Plan participant terminate before all shares are vested, unvested shares will be repurchased by the Company at a price per share equal to the original amount paid by the Plan participant. As of December 31, 2015, there were no unvested shares and 44,246 shares of Class A stock available for future grants under the Plan.our compensation program.
3Gives effect to 541,700 outstanding shares of restricted stock. As these shares are considered issued and outstanding upon grant, they are not included in the amounts reported in column (a).
108 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
71
Executive Compensation
![]()
When are shareholder proposals due for the 2017 Annual Meeting of Shareholders?
WHEN ARE SHAREHOLDER PROPOSALS DUE FOR THE 2021 ANNUAL MEETING OF SHAREHOLDERS?
A shareholder wishing to present a proposal at the 20172021 Annual Meeting of Shareholders must submit the proposal in writing and it must be received by the Company at its principal executive offices at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 by December 28, 2016,25, 2020, and must satisfy the other conditions established by the SEC, in order for such proposal to be considered for inclusion in the Company'sCompany’s proxy statement and form of proxy relating to that meeting.
Under our by-laws,Amended and Restated By-Laws, proposals of shareholders intended to be submitted for a formal vote (other than proposals to be included in our proxy statement) at the 20172021 Annual Meeting may be made only by a shareholder of record who has given notice of the proposal to the Secretary of the Company at our principal executive offices no earlier than 90 days and no later than 60 days prior to the meeting; provided that if less than 70 days'days’ notice or public disclosure of the date of the 20172021 Annual Meeting is given or made to shareholders, notice by the shareholder in order to be timely must be received no later than the close of business on the tenth day following the day on which such notice of the annual meeting was first mailed or such public disclosure of the annual meeting was made, whichever first occurs. The notice must contain certain information as specified in our by-laws.Amended and Restated By-Laws. Assuming our 20172021 Annual Meeting is held on June 9, 201711, 2021 in accordance with the Company'sCompany’s past practice, and at least 70 days'days’ notice or prior public disclosure of the date of the 20172021 Annual Meeting is given or made to shareholders, notice of such proposals would need to be given no earlier than March 11, 201713, 2021 and no later than April 10, 2017.12, 2021. Any proposal received outside of such dates will not be considered "timely"“timely” under the federal proxy rules for purposes of determining whether we may use discretionary authority to vote on such proposal.
What happens if multiple shareholders share an address?
WHAT HAPPENS IF MULTIPLE SHAREHOLDERS SHARE AN ADDRESS?
Applicable rules permit brokerage firms and the Company to send one Notice of Internet Availability of Proxy Materials (or one annual report, proxy statement, and Notice of Internet Availability of Proxy Materials in the case of shareholders who have elected to receive paper copies of our proxy materials) to multiple shareholders who share the same address under certain circumstances. This practice is known as "householding."“householding.” We believe that householding will provide greater convenience for our shareholders, as well as cost savings for us, by reducing the number of duplicate documents that are sent to your home. Consequently, we have implemented the practice of householding for shares held in "street name"“street name” and intend to deliver only one copy of the applicable proxy materials to
multiple shareholders sharing the same address. If you wish to receive separate copies of the proxy statement for the 20162020 Annual Meeting, the 20152019 Annual Report, or the Notice of Internet Availability of Proxy Materials, you may find these materials at our internet website (www.regeneron.com) or you may stop householding for your account and receive separate printed copies of these materials by contacting our Investor Relations Department, at Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, or by calling us at 914-847-7000, and these materials will be promptly delivered to you. If you hold shares registered in your name (sometimes called a shareholder of record), you can elect householding for your account by contacting us in the same manner described above. Any shareholder may stop householding for your account by contacting our Investor Relations Department at the address and/or phone number included above. If you revoke your consent, you will be removed from the householding program within 30 days of receipt of your revocation and each shareholder at your address will receive individual copies of our disclosure documents.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 109 / 109 |

| OTHER MATTERS |
Are there any other matters to be addressed at the Annual Meeting?
ARE THERE ANY OTHER MATTERS TO BE ADDRESSED AT THE ANNUAL MEETING?
We know of no other matters to be brought before the Annual Meeting, except as set forth in this proxy statement. If any other matter is properly presented at the Annual Meeting upon which a vote may properly be taken, shares represented by duly executed and timely submitted proxies will be voted on any such matter in accordance with the judgment of the persons named as proxies in the enclosed proxy card. Discretionary authority for them to do so is contained in the enclosed proxy card.
Who will pay the costs related to this proxy statement and the Annual Meeting?
The solicitation of proxies is being made on behalf of the Company and we will bear the costs of the solicitation. We will be responsible for paying for all expenses to prepare, print, and mail the proxy materials to shareholders. In accordance with the regulations of the SEC, we will make arrangements with brokerage houses and other custodians, nominees, and fiduciaries to send proxies and proxy materials to their principals and will reimburse them for their reasonable expenses in so doing. In addition to the solicitation by use of the mails and the Internet, certain of our officers, directors, and employees may solicit the return of proxies by telephone, e-mail or personal interviews.
72HOW CAN YOU RECEIVE A PRINTED COPY OF THE COMPANY’S 2019 ANNUAL REPORT?
Other Matters
How can you receive a printed copy of the Company's 2015 Annual Report?
Interested shareholders may obtain without charge a copy of our 20152019 Annual Report (without exhibits), which includes our audited financial statements for the fiscal year ended December 31, 2015,2019, required to be filed with the SEC, by making a written request to Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, Attention: Investor Relations, or by calling our Investor Relations Department at (914) 847-7000.
How do you elect to receive future proxy materials electronically?
HOW DO YOU ELECT TO RECEIVE FUTURE PROXY MATERIALS ELECTRONICALLY?
If you previously requested to receive proxy materials through the mail, or by means of an e-mail with links to the proxy materials and the proxy voting website, your election will remain in effect until you revoke it. Shareholders currently receiving paper copies of our proxy materials, and shareholders who received a paper copy of the Notice of Internet
Availability of Proxy Materials, may instead elect to receive all future proxy materials electronically through an e-mail with a link to these documents on the Internet. Receiving these documents online conserves resources, saves the Company the cost of producing and mailing documents to your home or business, and gives you an automatic link to the proxy voting site.
If your shares are registered in your name or you hold shares in the Company Stock Fund in the Company'sCompany’s 401(k) Savings Plan, to enroll in the electronic delivery service, vote your shares through the Internet atwww.proxyvote.comand, when prompted, indicate that you agree to receive or access shareholder communications electronically in future years. If your shares are not registered in your name, to enroll in the electronic delivery service, check the information provided to you by your bank or broker, or contact your bank or broker for instructions on how to elect to view future proxy statements and annual reports over the Internet.
110 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
73
Other Matters
![]()
Appendix A Note Regarding Forward-Looking Statements andNon-GAAPAnd Non-GAAP Financial Measures
This proxy statement includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. ("we," "us," "our," "Regeneron,"(where applicable, together with its subsidiaries, “Regeneron” or the "Company"“Company”), and actual events or results may differ materially from these forward-looking statements. Words such as "anticipate," "expect," "intend," "plan," "believe," "seek," "estimate,"“anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the impact of SARS-CoV-2 (the virus that has caused the COVID-19 pandemic) on Regeneron’s business and its employees, collaborators, suppliers, and other third parties on which Regeneron relies, Regeneron’s and its collaborators’ ability to continue to conduct research and clinical programs, Regeneron’s ability to manage its supply chain, net product sales of products marketed by Regeneron and/or its collaborators (collectively, “Regeneron’s Products”), and the global economy; the nature, timing, and possible success and therapeutic applications of our products,Regeneron’s Products and Regeneron’s product candidates and research and clinical programs now underway or planned, including without limitation EYLEA® EYLEA®(aflibercept) Injection, Praluent® Dupixent®(dupilumab), Libtayo®(cemiplimab), Praluent®(alirocumab) Injection, sarilumab, dupilumab,, Kevzara®(sarilumab), fasinumab, REGN 2222,evinacumab, REGN-EB3, garetosmab, pozelimab, Regeneron’s immuno-oncology programs (including its costimulatory bispecific portfolio), Regeneron’s COVID-19 antibody program and other earlier-stage programs, and the immuno-oncology program;use of human genetics in Regeneron’s research programs; the likelihood and timing of achieving any of Regeneron’s anticipated development and production milestones; unforeseen safety issues resulting from the administration of productsRegeneron’s Products and product candidates in patients, including serious complications or side effects in connection with the use of ourRegeneron’s Products and product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of our late-stageRegeneron’s product candidates and new indications for marketed products, including without limitation EYLEA®, Praluent®, sarilumab, dupilumab, fasinumab,Regeneron’s Products; the extent to which the results from the research and REGN 2222;development programs conducted by Regeneron or its collaborators may be replicated in other studies and lead to therapeutic applications; ongoing regulatory obligations and oversight impacting our marketed productsRegeneron’s Products (such as EYLEA®EYLEA, Dupixent, Libtayo, Praluent, and Praluent®)Kevzara), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict ourRegeneron’s ability to continue to develop or commercialize our productsRegeneron’s Products and product candidates; competing drugs and product candidates that may be superior to our productsRegeneron’s Products and product candidates; uncertainty of market acceptance and commercial success of our productsRegeneron’s Products and product candidates; ourthe ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; ourthe ability of Regeneron to meet any of its sales or other financial projections or guidance, and changes to the assumptions underlying those projections or guidance; the potential for any license or collaboration agreement, including ourRegeneron’s agreements with Sanofi, Bayer, and Bayer HealthCare LLC,Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success; and risks associated with third party intellectual property of others and
pending or future litigation relating thereto.thereto (including without limitation the patent litigation and other related proceedings relating to Dupixent and Praluent), other litigation and other proceedings and government investigations relating to the Company and/or its operations, the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in ourRegeneron’s filings with the U.S. Securities and Exchange Commission, including our Annual Report onits Form 10-K for the fiscal year ended December 31, 2015 (filed with the Securities and Exchange Commission on February 11, 2016),2019, including in the section thereof captioned "Item“Item 1A. Risk Factors."” Any forward-looking statements are made based on management'smanagement’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. We doRegeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 111 / 111 |

OTHER MATTERS / APPENDIX A
This proxy statement uses non-GAAP net income and non-GAAP net income per share, which is aare financial measuremeasures that isare not calculated in accordance with U.S. Generally Accepted Accounting Principles ("GAAP"(“GAAP”). We believeThese non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the estimated income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that the presentation of this non-GAAP measure is useful to investors because it excludes (i) non-cash share-based compensation expense, which fluctuatesfluctuate from period to period based on factors that are not within the Company'sCompany’s control such(such as the Company'sCompany’s stock price on the dates share-based grants are issued; (ii) the incremental charge recorded in the third quarter of 2014 related to the issuance of the final IRS regulations that provide guidance on the annual fee imposed by the Patient Protection and Affordable Care Act (the final IRS regulations differed from the temporary regulations issued in 2011 which resulted in the recognition of a catch-up adjustment); (iii) non-cash interest expense related to the Company's convertible senior notes, since this is not deemed useful in evaluating the Company's operating performance; (iv) loss on extinguishment of debt, since this non-cash charge is based on factorsissued) or items that are not within the Company's control;associated with normal, recurring operations (such as changes in applicable laws and (v) income tax expense for 2014, which was principally a non-cash expense due primarily to utilization of net operating loss and tax credit carryforwards, and deductions related to employee stock option exercises. In 2015, income tax expense adjustments consider the tax effect of reconciling items and an adjustment from GAAP tax expense to the amount of taxes that are paid or payable in cash in respect of the current period. As there is a significant difference between the Company's effective tax rate and actual cash income taxes paid or payable, GAAP income tax expense is not deemed useful in evaluating the Company's operating performance.regulations). Management uses these non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial
A-1
Appendix A Note Regarding Forward-Looking Statements and Non-GAAP Financial Measures
and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company'sCompany’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other
companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP. A reconciliation of ourthe Company’s historical GAAP to non-GAAP results is included below.
Reconciliation of GAAP Net Income to Non-GAAP Net Income
(Unaudited)(In thousands) (In millions, except per share data)
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| GAAP net income | $ | 2,115.8 | $ | 2,444.4 | ||||
| Adjustments: | ||||||||
| R&D: Non-cash share-based compensation expense | 250.4 | 229.0 | ||||||
| R&D: Up-front payments related to license and collaboration agreements | 430.0 | — | ||||||
| SG&A: Non-cash share-based compensation expense | 167.7 | 169.2 | ||||||
| SG&A: Restructuring-related expenses | 35.2 | — | ||||||
| SG&A: Litigation contingencies | 70.0 | 30.0 | ||||||
| COGS and COCM: Non-cash share-based compensation expense | 46.2 | 29.2 | ||||||
| Other income/expense: (Gains) losses on investments in equity securities | (118.3 | ) | 41.9 | |||||
| Income tax effect of reconciling items above | (169.9 | ) | (92.1 | ) | ||||
| Income tax expense: Impact of sale of assets between foreign subsidiaries | — | (162.1 | ) | |||||
| Income tax expense: Adjustment to previously recorded charge related to enactment of U.S. Tax Reform Act | — | (68.0 | ) | |||||
| Non-GAAP net income | $ | 2,827.1 | $ | 2,621.5 | ||||
| GAAP net income per share—basic | $19.38 | $22.65 | ||||||
| GAAP net income per share—diluted | $18.46 | $21.29 | ||||||
| Non-GAAP net income per share—basic | $25.89 | $24.30 | ||||||
| Non-GAAP net income per share—diluted | $24.67 | $22.84 | ||||||
| Shares used in calculating: | ||||||||
| Net income per share—basic | 109.2 | 107.9 | ||||||
| Net income per share—diluted | 114.6 | 114.8 | ||||||
112 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
| | Year ended December 31, | |||||
| | | | | | | | |
| | 2015 | | 2014* | ||||
GAAP net income | $ | 636,056 | $ | 338,126 | |||
Adjustments: | | | |||||
R&D: Non-cash share-based compensation expense | | 255,708 | | 184,347 | |||
SG&A: Non-cash share-based compensation expense | | 193,026 | | 134,715 | |||
SG&A: Branded Prescription Drug Fee incremental charge | | — | | 40,600 | |||
COGS and COCM: Non-cash share-based compensation expense | | 10,315 | | 2,688 | |||
Interest expense: Non-cash interest related to convertible senior notes | | 2,818 | | 17,821 | |||
Other expense: Loss on extinguishment of debt | | 18,861 | | 33,469 | |||
Non-cash income taxes | | 287,110 | | 423,109 | |||
| | | | | | | | |
Non-GAAP net income | $ | 1,403,894 | $ | 1,174,875 | |||
| | | | | | | | |
*Certain revisions have been made

Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan
1. Purpose; Establishment
The Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (the “Plan”) is intended to promote the interests of the Company (as defined below) and its shareholders by providing officers, other employees of the Company (including directors who are also employees of the Company) and consultants to the amountsCompany with appropriate incentives and rewards to encourage them to enter into and continue in the employ or service of the Company and to acquire a proprietary interest in the long-term success of the Company; to compensate the Company’s nonemployee directors and provide incentives to such nonemployee directors that are directly linked to increases in stock value; and to reward the performance of individual officers, other employees, consultants and nonemployee directors of the Company in fulfilling their personal responsibilities for long-term achievements.
The Plan was originally reportedadopted and approved by the Board of Directors (defined below) on April 4, 2014 and became effective as of such date, subject to the approval of the shareholders of the Company, which was obtained on June 13, 2014 (the “Original Plan”). The initial amendment and restatement of the Plan was adopted and approved by the Board of Directors on April 6, 2017 and became effective as of such date, subject to the approval of the shareholders of the Company, which was obtained on June 9, 2017 (the “First Restatement”). This second amendment and restatement of the Plan was adopted and approved by the Board of Directors on April 3, 2020 and became effective as of such date, subject to the approval of the shareholders of the Company.
2. Definitions
As used in the Plan, in addition to the terms defined elsewhere in the Plan, the following definitions shall have the respective meanings indicated below:
| (a) | “Affiliate”means any entity if, at the time of granting of an Award (A) the Company, directly or indirectly, owns at least 50% of the combined voting power of all classes of stock of such entity or at least 50% of the ownership interests in such entity or (B) such entity, directly or indirectly, owns at least 50% of the combined voting power of all classes of stock of the Company. |
| (b) | “Agreement”shall mean the written agreement between the Company and a Participant evidencing an Award (or, if no written agreement is entered into, a notice issued by the Company evidencing an Award). |
| (c) | “Award”shall mean any Option, Restricted Stock, Phantom Stock, Stock Appreciation Right, Stock Bonus or Other Award granted pursuant to the terms of the Plan. |
| (d) | “Beneficial Owner”and“Beneficially Owned”shall have the meaning afforded to such terms in accordance with Rule 13d-3 under the Exchange Act. |
| (e) | “Board of Directors”shall mean the Board of Directors of Regeneron Pharmaceuticals, Inc. |
| (f) | “Change in Control”shall be deemed to have occurred if the event set forth in any one of the following paragraphs shall have occurred after the Effective Date: |
| (1) | any Person is or becomes the Beneficial Owner, directly or indirectly, of securities of the Company (not including in the securities Beneficially Owned by such Person any securities acquired directly from the Company) representing 20% or more of the Company’s then outstanding securities, excluding any Person who is an officer or director of the Company or who becomes such a Beneficial Owner in connection with a transaction described in clause (A) of paragraph (3) below; or |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 113 / 113 |
OTHER MATTERS / APPENDIX B
| (2) | the following individuals cease for any reason to constitute a majority of the number of directors then serving: individuals who, on the Effective Date, constitute the Board of Directors and any new director (other than a director whose initial assumption of office is in connection with an actual or threatened election contest, including but not limited to a consent solicitation, relating to the election of directors of the Company) whose appointment or election by the Board of Directors or nomination for election by the Company’s shareholders was approved or recommended by a vote of at least two-thirds (2/3) of the directors then still in office who either were directors on the Effective Date or whose appointment, election or nomination for election was previously so approved or recommended; or | |
| (3) | there is consummated a merger or consolidation of the Company or any directly or indirectly held subsidiary of the Company with any entity other than (A) a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior to such merger or consolidation continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or any parent thereof) at least 60% of the combined voting power of the voting securities of the Company or such surviving entity or any parent thereof outstanding immediately after such merger or consolidation, or (B) a merger or consolidation effected to implement a recapitalization of the Company (or similar transaction) in which no Person is or becomes the Beneficial Owner, directly or indirectly, of securities of the Company (not including in the securities Beneficially Owned by such Person any securities acquired directly from the Company) representing 20% or more of the combined voting power of the Company’s then outstanding securities; or | |
| (4) | the shareholders of the Company approve a plan of complete liquidation or dissolution of the Company or there is consummated a sale or disposition by the Company of all or substantially all of the Company’s assets, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity at least 60% of the combined voting power of the voting securities of which are owned by Persons in substantially the same proportions as their ownership of the Company immediately prior to such sale or disposition. | |
| (g) | “Cause”shall have the meaning set forth in the Agreement, or if no such definition is set forth in the Agreement, shall be determined by the Committee in its reasonable discretion. |
| (h) | “Code”shall mean the Internal Revenue Code of 1986, as amended from time to time, and any regulations promulgated thereunder. |
| (i) | “Committee”shall mean, at the discretion of the Board of Directors, the full Board of Directors or a committee of the Board of Directors, which shall consist of two or more persons, each of whom, unless otherwise determined by the Board of Directors, is a “Non-Employee Director” within the meaning of Rule 16b-3 and who meets such other applicable independence standards imposed by law, regulation or listing standard. |
| (j) | “Company”shall mean Regeneron Pharmaceuticals, Inc., a New York corporation, and, where appropriate (but specifically not for purposes of Section 2(f) hereof), each of its Affiliates. |
| (k) | “Company Stock”shall mean the common stock of the Company, par value $0.001 per share. |
| (l) | “Effective Date”shall mean April 3, 2020. |
| (m) | “Exchange Act”shall mean the Securities Exchange Act of 1934, as amended from time to time. |
| (n) | “Fair Market Value”of a share of Company Stock, as of a date of determination, shall mean (1) the average of the high and low sales price per share of Company Stock on the national securities exchange or national market system on which such stock is principally traded on such date or, if such date is not a trading day, on the last preceding date on which there was a sale of such stock on such exchange, or (2) if the shares of Company Stock are not then listed on a national securities exchange or national market system, or the value of such shares is not otherwise determinable, such value as determined by the Committee in good faith. |
| (o) | “Incentive Stock Option”shall mean an Option that is an “incentive stock option” within the meaning of Section 422 of the Code, or any successor provision, and that is designated by the Committee as an Incentive Stock Option. |
| (p) | “Nonemployee Director”shall mean a member of the Board of Directors who is not an employee of the Company. |
114 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
| (q) | “Nonqualified Stock Option”shall mean an Option other than an Incentive Stock Option. |
| (r) | “Option”shall mean an option to purchase shares of Company Stock granted pursuant to Section 7 hereof. |
| (s) | “Other Award”shall mean an award granted pursuant to Section 11 hereof. |
| (t) | “Participant”shall mean an employee or consultant of the Company or a Nonemployee Director to whom an Award is granted pursuant to the Plan, or upon the death of such individual, his or her successors, heirs, executors and administrators, as the case may be. |
| (u) | “Person”shall have the meaning set forth in Section 3(a)(9) of the Exchange Act, as modified and used in Sections 13(d) and 14(d) thereof, except that such term shall not include (1) the Company, (2) a trustee or other fiduciary holding securities under an employee benefit plan of the Company, (3) an underwriter temporarily holding securities pursuant to an offering of such securities, or (4) an entity owned, directly or indirectly, by the shareholders of the Company in substantially the same proportions as their ownership of stock of the Company. |
| (v) | “Phantom Stock”shall mean the right, granted pursuant to Section 9 hereof, to receive in cash or shares the Fair Market Value of a share of Company Stock. |
| (w) | “Restricted Stock”shall mean a share of Company Stock which is granted pursuant to the terms of Section 8 hereof and which is subject to the restrictions set forth in Section 8(c) hereof and/or Section 8(d) hereof. |
| (x) | “Rule 16b-3”shall mean the Rule 16b-3 promulgated under the Exchange Act, as amended from time to time. |
| (y) | “Securities Act”shall mean the Securities Act of 1933, as amended from time to time. |
| (z) | “Stock Appreciation Right”shall mean the right granted to a Participant pursuant to Section 7(h) hereof. |
| (aa) | “Stock Bonus”shall mean a bonus payable in shares of Company Stock granted pursuant to Section 10 hereof. |
| (bb) | “Subsidiary”shall mean a “subsidiary corporation” within the meaning of Section 424(f) of the Code. |
| (cc) | “Substitute Awards”shall mean Awards granted or shares of Company Stock issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines. |
| (dd) | “Vesting Date”shall mean the date established by the Committee on which a share of Restricted Stock or Phantom Stock vests. |
3. Stock Subject to the Plan
| (a) | Shares Available for Awards |
| The shares of Company Stock that may be issued with respect to Awards made under the Plan may be authorized but unissued Company Stock or authorized and issued Company Stock held in the Company treasury (including authorized and issued shares of Company Stock acquired or purchased by the Company and held by the Company as treasury shares). | |
| Subject to the subsequent provisions of this Section 3, including the adjustment provisions contained herein, the maximum number of shares of Company Stock that may be delivered pursuant to Awards made under the Plan shall equal the sum of: (1) 22,150,052 shares of Company Stock; (2) the shares of Company Stock subject to outstanding awards under the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan (the “2000 Plan”) as of April 17, 2014, (3) the shares of Company Stock subject to outstanding awards under the Original Plan as of April 13, 2017 and (4) the shares of Company Stock subject to outstanding awards under the First Restatement as of April 14, 2020, in each case that (i) remain unissued upon the cancellation, surrender, exchange or termination of any such award for any reason whatsoever or are forfeited, (ii) are delivered (or deliverable) by a participant in the 2000 Plan, the Original Plan or the First Restatement (as applicable) pursuant to an exercise of an option to purchase Company Stock and received (or retained) by the Company (whether by actual delivery, attestation |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 115 / 115 |
OTHER MATTERS / APPENDIX B
| or otherwise) in payment of the option exercise price (for this avoidance of doubt, for the purpose of this clause (ii), in the case of Options where the exercise price is paid by the Company’s retention of shares of Company Stock that would otherwise be distributed to the Participant upon exercise of the Option, the retained shares are considered deliverable by the Participant and become available for future issuance) or (iii) are received (or retained) by the Company (whether by actual delivery, attestation or otherwise) in connection with the exercise, vesting or delivery of an award granted under the 2000 Plan, the Original Plan or the First Restatement (as applicable) in respect of tax withholding or other similar tax obligation; and (3) any shares of Company Stock that again become available for Awards pursuant to Section 3(e) hereof. Notwithstanding the foregoing, the maximum number of shares of Company Stock that may be issued pursuant to Incentive Stock Options during the term of the Plan shall be 22,150,052 shares. | |
| (b) | [Reserved] |
| (c) | Adjustment for Change in Capitalization |
| In the event that any dividend or other distribution is declared (whether in the form of cash, Company Stock or other property), or there occurs any recapitalization, Company Stock split, reverse Company Stock split, reorganization, merger, consolidation, spin-off, combination, repurchase or share exchange, or other similar corporate transaction or event, unless the Committee determines that it is otherwise inappropriate, (1) the number and kind of shares of Company Stock which may thereafter be issued in connection with Awards, (2) the number and kind of shares of Company Stock issued or issuable in respect of outstanding Awards, (3) the exercise price, grant price or purchase price relating to any Award, (4) the maximum number of shares of Company Stock that may be issued pursuant to Incentive Stock Options during the term of the Plan and (5) the maximum number of shares subject to Awards which may be awarded to any Participant during any tax year of the Company shall be equitably adjusted as necessary to prevent the dilution or enlargement of the rights of Participants without change in the aggregate purchase price; provided that, with respect to Incentive Stock Options, such adjustment shall be made in accordance with Section 424 of the Code. | |
| (d) | Adjustment for Change or Exchange of Shares for Other Consideration |
| In the event the outstanding shares of Company Stock shall be changed into or exchanged for any other class or series of capital stock or cash, securities or other property pursuant to a recapitalization, reclassification, merger, consolidation, combination or similar transaction (“Transaction”), then, unless otherwise determined by the Committee in its sole and absolute discretion, (1) each Option shall thereafter become exercisable for the number and/or kind of capital stock, and/or the amount of cash, securities or other property so distributed, into which the shares of Company Stock subject to the Option would have been changed or exchanged had the Option been exercised in full prior to such transaction, provided that, if the kind or amount of capital stock or cash, securities or other property received in such transaction is not the same for each outstanding share, then the kind or amount of capital stock or cash, securities or other property for which the Option shall thereafter become exercisable (or the other Award shall thereafter represent) shall be the kind and amount so receivable per share by a plurality of the shares of Company Stock, and provided further that, if necessary, the provisions of the Option shall be appropriately adjusted so as to be applicable, as nearly as may reasonably be, to any shares of capital stock, cash, securities or other property thereafter issuable or deliverable upon exercise of the Option, and (2) each Award that is not an Option and that is not automatically changed in connection with the Transaction shall represent the number and/or kind of shares of capital stock, and/or the amount of cash, securities or other property so distributed, into which the number of shares of Company Stock covered by the Award would have been changed or exchanged had they been held by a shareholder of the Company. | |
| (e) | Reuse of Shares |
| The following shares of Company Stock shall again become available for Awards: (1) any shares subject to an Award that remain unissued upon the cancellation, surrender, exchange or termination of such award for any reason whatsoever and any shares of Restricted Stock forfeited; (2) any shares delivered (or deliverable) to the Participant pursuant to an Option exercise and received (or retained) by the Company (whether by actual delivery, attestation or otherwise) in payment of the Option Exercise Price upon a Participant’s exercise of an Option as permitted under Section 7(c)(3) hereof (for the avoidance of doubt, for the purpose of this clause (2), in the case of Options where the exercise price is paid by the Company’s retention of shares of Company Stock that would otherwise be distributed |
116 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
| to the Participant upon exercise of the Option, the retained shares are considered deliverable by the Participant and become available for future issuance); and (3) any shares received (or retained) by the Company (whether by actual delivery, attestation or otherwise) in connection with the exercise, vesting or delivery of an Award in respect of tax withholding or other similar tax obligations. | |
| (f) | Substitute Awards |
| Substitute Awards shall not reduce the shares of Company Stock authorized for grant under the Plan or the applicable limitations on grants to a Participant under Section 3(b) hereof, nor shall shares of Company Stock subject to a Substitute Award be added to the shares of Company Stock available for Awards under the Plan as provided in Section 3(e) hereof. Additionally, in the event that a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines has shares available under a pre-existing plan approved by shareholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of such pre-existing plan (as adjusted, to the extent appropriate, using the exchange ratio or other adjustment or valuation ratio or formula used in such acquisition or combination to determine the consideration payable to the holders of common stock of the entities party to such acquisition or combination) may be used for Awards under the Plan and shall not reduce the shares of Company Stock authorized for grant under the Plan; and shares of Company Stock subject to such Awards shall not be added to the shares of Company Stock available for Awards under the Plan as provided in Section 3(e) hereof; provided that Awards using such available shares shall not be made after the date awards or grants could have been made under the terms of the pre-existing plan, absent the acquisition or combination, and shall only be made to individuals who are eligible to receive such Awards under the rules of the principal U.S. national securities exchange on which the shares of Company Stock are listed. | |
4. Administration of the Plan
The Plan shall be administered by the Committee. The Committee shall have the authority in its sole discretion, subject to and not inconsistent with the express provisions of the Plan, to administer the Plan and to exercise all the powers and authorities either specifically granted to it under the Plan or necessary or advisable in the administration of the Plan, including, without limitation, the authority to grant Awards; to determine the persons to whom and the time or times at which Awards shall be granted; to determine the type and number of Awards to be granted, the number of shares of Company Stock to which an Award may relate and the terms, conditions, restrictions and performance criteria relating to any Award; to determine whether, to what extent and under what circumstances an Award may be settled, canceled, forfeited, exchanged or surrendered; to make adjustments in the performance goals in recognition of unusual or nonrecurring events affecting the Company or the financial statements of the Company, or in response to changes in applicable laws, regulations or accounting principles; to construe and interpret the Plan and any Award; to prescribe, amend and rescind rules and regulations relating to the Plan; to determine the terms and provisions of Agreements; and to make all other determinations deemed necessary or advisable for the year ended December 31, 2014 (see Note 1administration of the Plan.
The Committee may, in its sole and absolute discretion, without amendment to the Company's audited financial statementsPlan, (a) accelerate the date on which any Option granted under the Plan becomes exercisable, waive or amend the operation of Plan provisions respecting exercise after termination of employment or otherwise adjust any of the terms of such Option, and (b) accelerate the Vesting Date, or waive any condition imposed hereunder, with respect to any share of Restricted Stock, Phantom Stock or other Award or otherwise adjust any of the terms applicable to any such Award. Except pursuant to the operation of Section 3(c) hereof or Section 3(d) hereof, the Committee shall not have the authority to decrease the exercise price of Options granted under the Plan.
The Committee may delegate to one or more of the Company’s executive officers (or, in the case of ministerial duties only, other employees) all or any portion of the Committee’s authority, powers, responsibilities and administrative duties under the Plan, with such conditions and limitations as are required under applicable law or as the Committee may otherwise prescribe; provided, however, that only the Committee is authorized to grant Awards to, or make decisions or interpretations with respect to Awards granted to, Participants who are subject to potential liability under Section 16(b) of the Exchange Act with respect to transactions involving equity securities of the Company. A record of all actions taken by any executive officer (or other employee) to whom the Committee has delegated a portion of its powers or responsibilities shall periodically be filed with the minutes of the meetings of the Committee and shall be made available for review by the Committee upon request.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 117 / 117 |
OTHER MATTERS / APPENDIX B
5. Eligibility
The persons who shall be eligible to receive Awards pursuant to the Plan shall be such employees of the Company (including officers of the Company, whether or not they are directors of the Company), Nonemployee Directors and nonemployee service providers and consultants to the Company, in each case as the Committee shall select from time to time. The grant of any Award hereunder at any time to any such Nonemployee Director, employee, service provider or consultant shall not entitle such person to a grant of an Award at any future time.
6. Awards Under the Plan; Agreement; Performance Goals
The Committee may grant Options, shares of Restricted Stock, Phantom Stock, Stock Appreciation Right, Stock Bonuses and Other Awards in such amounts and with such terms and conditions as the Committee shall determine, subject to the provisions of the Plan.
Each Award granted under the Plan (except an unconditional Stock Bonus) shall be evidenced by an Agreement which shall contain such provisions as the Committee may in its sole discretion deem necessary or desirable which are not in conflict with the terms of the Plan. By accepting an Award, a Participant thereby agrees that the award shall be subject to all of the terms and provisions of the Plan and the applicable Agreement.
Any Company Stock required to be issued to a Participant under the Plan shall be evidenced in such manner as the Committee may in its sole discretion deem appropriate, including book-entry registration or delivery of stock certificates. References in the Plan or in any Agreement to stock certificates shall be deemed to refer to book-entry registration in the case of any Company Stock evidenced in such manner and the provisions regarding stock certificates shall be applicable to Company Stock evidenced in book-entry formmutatis mutandis.
Notwithstanding anything to the contrary contained in the Plan, any Award granted under the Plan may be (but is not required to be) subject to vesting based on the attainment by the Company of performance goals established by the Committee.
7. Options and Stock Appreciation Rights
| (a) | Identification of Options |
| Each Option shall be clearly identified in the applicable Agreement as either an Incentive Stock Option or a Nonqualified Stock Option. | |
| (b) | Exercise Price |
| Each Agreement with respect to an Option shall set forth the amount (the “Option Exercise Price”) payable by the grantee to the Company upon exercise of the Option. The Option Exercise Price per share shall be determined by the Committee; provided, however, that (other than in connection with Substitute Awards) the Option Exercise Price shall in no event be less than the Fair Market Value of a share of Company Stock on the date the Option is granted. Without limitation of the authority set forth in Section 3(c) hereof or Section 3(d) hereof, no Option shall be settled, canceled, forfeited, exchanged or surrendered in exchange or otherwise in consideration for a new Option with an Option Exercise Price that is less than that of such settled, canceled, forfeited, exchanged or surrendered Option, nor shall any Option be settled, canceled, forfeited, exchanged or surrendered in exchange or otherwise in consideration for a cash payment from the Company in excess of the difference between the Option Exercise Price of such Option and the Fair Market Value of the shares of Company Stock subject to such Option on the day of such payment. | |
| (c) | Term and Exercise of Options |
| (1) | Unless the applicable Agreement provides otherwise, an Option shall become cumulatively exercisable as to 25% of the shares covered thereby on each of the first, second, third, and fourth anniversaries of the date of grant. The Committee shall determine the expiration date of each Option; provided, however, that no Incentive Stock Option shall be exercisable more than ten (10) years after the date of grant. | |
| (2) | To the extent that an Option to purchase shares is not exercised by a Participant when it becomes initially exercisable, it shall not expire but carry forward and shall be exercisable until its expiration or as provided by Section 7(e) hereof. If any Option is exercisable in the amount of one hundred (100) or more full shares of Company stock, the Company shall not be obligated to permit the partial exercise of such exercisable Option for less than one hundred (100) full shares. |
118 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
| (3) | An Option shall be exercised by delivering notice as specified in the Agreement on the form of notice provided by the Company. Payment for the exercise price of the shares of Company Stock purchased upon the exercise of an Option shall be made on the effective date of such exercise by one or a combination of the following means: (A) in cash or by personal check, certified check, bank cashier’s check or wire transfer; (B) in shares of Company Stock owned by the Participant for at least six months prior to the date of exercise and valued at their Fair Market Value on the effective date of such exercise; or (C) by any such other methods as the Committee may from time to time authorize (which shall include the use of a broker-assisted cashless exercise procedure and the payment of the exercise price by authorizing the Company to retain shares of Company Stock that otherwise would be distributed to the Participant upon exercise of the Option). In the case of a Participant who is subject to Section 16 of the Exchange Act, the Company may require that the method of making such payment be in compliance with Section 16 of the Exchange Act and the rules and regulations thereunder. Any payment in shares of Company Stock shall be effected by the delivery of such shares to the Secretary of the Company, duly endorsed in blank or accompanied by stock powers duly executed in blank, together with any other documents and evidences as the Secretary of the Company shall require. | |
| (4) | Certificates (if any) for shares of Company Stock purchased upon the exercise of an Option shall be issued in the name of or for the account of the Participant or other person entitled to receive such shares, and delivered to the Participant or such other person as soon as practicable following the effective date on which the Option is exercised. | |
| (5) | Notwithstanding the foregoing, an Agreement may provide that if on the last day of the term of an Option the Fair Market Value of one share of Company Stock exceeds the option price per share of Company Stock, the Participant has not exercised the Option (or a tandem Stock Appreciation Right, if applicable) and the Option has not expired, the Option shall be deemed to have been exercised by the Participant on such day with payment made by withholding shares of Company Stock otherwise issuable in connection with the exercise of the Option. In such event, the Company shall deliver to the Participant the number of shares of Company Stock for which the Option was deemed exercised, less the number of shares of Company Stock required to be withheld for the payment of the total purchase price and required withholding taxes; provided, however, any fractional shares of Company Stock shall be settled in cash. | |
| (d) | Limitations on Incentive Stock Options |
| (1) | To the extent that the aggregate Fair Market Value of shares of Company Stock with respect to which Incentive Stock Options are exercisable for the first time by a Participant during any calendar year under the Plan and any other stock option plan of the Company or a Subsidiary shall exceed $100,000, such Options shall be treated as Nonqualified Stock Options. Such Fair Market Value shall be determined as of the date on which each such Incentive Stock Option is granted. | |
| (2) | No Incentive Stock Option may be granted to an individual if, at the time of the proposed grant, such individual owns (or is deemed to own under the Code) stock possessing more than ten percent of the total combined voting power of all classes of stock of the Company unless (A) the exercise price of such Incentive Stock Option is at least 110% of the Fair Market Value of a share of Company Stock at the time such Incentive Stock Option is granted, and (B) such Incentive Stock Option is not exercisable after the expiration of five years from the date such Incentive Stock Option is granted. | |
| (e) | Effect of Termination of Employment |
| (1) | In the event that the employment of a Participant with the Company shall terminate for any reason other than (A) Cause or (B) death, the Options granted to such Participant, to the extent that they are exercisable at the time of such termination, shall remain exercisable for such period as may be provided in the Agreement (or as may be provided by the Committee), but in no event following the expiration of its term. The treatment of any Option that remains unexercisable as of the date of termination shall be as set forth in the Agreement (or as may be otherwise determined by the Committee). |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 119 / 119 |
OTHER MATTERS / APPENDIX B
| (2) | In the event that the employment of a Participant with the Company shall terminate on account of the death of the Participant, all Options granted to such Participant that remain outstanding as of the date of death shall become fully exercisable and shall remain exercisable by the Participant’s legal representatives, heirs or legatees for such period as may be provided in the Agreement (or as otherwise may be determined by the Committee), but in no event following the expiration of their respective terms. Unless the applicable Agreement provides otherwise, cessation of active employment or service due to commencement of long-term disability as determined by the Committee shall not be deemed to constitute a termination of employment or service for purposes of the Plan, and during the continuance of such long-term disability the individual shall be deemed to continue active employment or service with the Company; provided, however, that the Committee may in its sole discretion determine that a Participant’s long-term disability constitutes a permanent disability and may deem such permanent disability to be a termination of employment or service for any or all purposes under this Plan. | |
| (3) | In the event of the termination of a Participant’s employment for Cause, all outstanding Options granted to such Participant shall expire at the commencement of business on the date of such termination. | |
| (f) | Acceleration of Exercise Date Upon Change in Control |
| The Committee in its sole and absolute discretion may provide, either at the time of grant as provided in the Agreement or thereafter, that upon the occurrence of a Change in Control, an Option granted under the Plan and outstanding at such time shall (1) become immediately exercisable in whole or in part (in which case the Committee shall determine the period during which such Option shall remain exercisable), and/or (2) be canceled in exchange for the right to receive property equivalent in value to such Option, as determined by the Committee. | |
| (g) | Leave of Absence |
| In the case of any Participant on an approved leave of absence, the Committee may make such provision respecting the continuance of the Options held by such Participant while in the employ or service of the Company as it may deem equitable, except that in no event may an Option be exercised after its expiration. | |
| (h) | Stock Appreciation Rights |
| (1) | The Committee may grant Stock Appreciation Rights (a) in tandem with all or part of any Option granted under the Plan or at any subsequent time during the term of such Option, (b) in tandem with all or part of any Award (other than an Option) granted under the Plan or at any subsequent time during the term of such Award or (c) without regard to any Option or other Award, in each case upon such terms and conditions as the Committee may establish in its sole discretion. | |
| (2) | Stock Appreciation Rights shall be subject to such terms and conditions, not inconsistent with the provisions of the Plan, as shall be determined from time to time by the Committee, including the following: | |
| (A) | Unless the applicable Agreement provides otherwise, a Stock Appreciation Right shall become cumulatively exercisable as to 25% of the shares of Company Stock covered thereby on each of the first, second, third, and fourth anniversaries of the date of grant. The Committee shall determine the expiration date of each Stock Appreciation Right. | |
| (B) | Upon the exercise of a Stock Appreciation Right, the holder shall have the right to receive the excess of (i) the Fair Market Value of one share of Company Stock on the date of exercise (or such amount less than such Fair Market Value as the Committee shall so determine at any time during a specified period before the date of exercise) over (ii) the grant price of the Stock Appreciation Right. | |
| (C) | The Committee shall determine in its sole discretion whether payment on exercise of a Stock Appreciation Right shall be made in cash, in whole shares of Company Stock or other property, or any combination thereof. | |
| (D) | The terms and conditions of Stock Appreciation Rights need not be the same with respect to each recipient. |
120 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
| (E) | The Committee may impose such other terms and conditions on the exercise of any Stock Appreciation Right as it shall deem appropriate. A Stock Appreciation Right shall (i) have a grant price per share of Company Stock of no less than the Fair Market Value of one share of Company Stock on the date of grant or, if applicable, on the date of grant of an Option with respect to a Stock Appreciation Right granted in exchange for or in tandem with, but subsequent to, the Option (subject to the requirements of Section 409A of the Code) except in the case of Substitute Awards or in connection with an adjustment provided in Section 3(c) hereof. | |
| (F) | An Agreement may provide that if on the last day of the term of a Stock Appreciation Right the Fair Market Value of one share of Company Stock exceeds the grant price per share of Company Stock of the Stock Appreciation Right, the Participant has not exercised the Stock Appreciation Right or the tandem Option (if applicable), and the Stock Appreciation Right has not otherwise expired, the Stock Appreciation Right shall be deemed to have been exercised by the Participant on such day. In such event, the Company shall make payment to the Participant in accordance with this Section, reduced by the number of shares of Company Stock (or cash) required for withholding taxes; any fractional share of Company Stock shall be settled in cash. | |
Without the approval of the Company’s shareholders, other than pursuant to Section 3(e) hereof, the Committee shall not (i) reduce the grant price of any Stock Appreciation Right after the date of grant, (ii) cancel any Stock Appreciation Right when the grant price per Share exceeds the Fair Market Value of one share of Company Stock in exchange for cash or another Award (other than in connection with a Change in Control), or (iii) take any other action with respect to a Stock Appreciation Right that would be treated as a repricing under the rules and regulations of the principal U.S. national securities exchange on which the shares of Company Stock are listed.
8. Restricted Stock
| (a) | Price |
| At the time of the grant of shares of Restricted Stock, the Committee shall determine the price, if any, to be paid by the Participant for each share of Restricted Stock subject to the Award. | |
| (b) | Vesting Date |
| Subject to Section 31 hereof, at the time of the grant of shares of Restricted Stock, the Committee shall establish a Vesting Date or Vesting Dates with respect to such shares. The Committee may divide such shares into classes and assign a different Vesting Date for each class. Provided that all conditions to the vesting of a share of Restricted Stock imposed pursuant to Section 8(c) hereof are satisfied, and except as provided in Section 8(h), upon the occurrence of the Vesting Date with respect to a share of Restricted Stock, such share shall vest and the restrictions of Section 8(d) hereof shall lapse. | |
| (c) | Conditions to Vesting |
| Subject to Section 31 hereof, at the time of the grant of shares of Restricted Stock, the Committee may impose such restrictions or conditions to the vesting of such shares as it, in its absolute discretion, deems appropriate. | |
| (d) | Restrictions on Transfer Prior to Vesting |
| Prior to the vesting of a share of Restricted Stock, no transfer of a Participant’s rights with respect to such share, whether voluntary or involuntary, by operation of law or otherwise, shall be permitted. Immediately upon any attempt to transfer such rights, such share, and all of the rights related thereto, shall be forfeited by the Participant. | |
| (e) | Dividends on Restricted Stock |
| The Committee in its discretion may require that any dividends paid on shares of Restricted Stock be held in escrow until all restrictions on such shares have lapsed. |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 121 / 121 |
OTHER MATTERS / APPENDIX B
| (f) | Issuance of Certificates |
| (1) | Reasonably promptly after the date of grant with respect to shares of Restricted Stock, the Company shall cause to be issued a stock certificate, registered in the name of or for the account of the Participant to whom such shares were granted, evidencing such shares. Each such stock certificate shall bear a legend substantially in the following form: | |
| The transferability of this certificate and the shares of stock represented hereby are subject to the restrictions, terms and conditions (including forfeiture provisions and restrictions against transfer) contained in the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan and an Agreement entered into between the registered owner of such shares and the Company. A copy of the Plan and the Agreement is on file in the office of the Secretary of the Company, 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707. | ||
| Such legend shall not be removed until such shares vest pursuant to the terms hereof. Alternatively, in its sole discretion, the Company may document ownership of such shares in book-entry form. | ||
| (2) | Each certificate (if any) issued pursuant to this Section 8(f) hereof, together with the stock powers relating to the shares of Restricted Stock evidenced by such certificate, shall be held by the Company unless the Committee determines otherwise. | |
| (g) | Consequences of Vesting |
| Upon the vesting of a share of Restricted Stock pursuant to the terms hereof, the restrictions of Section 8(d) hereof shall lapse with respect to such share. Reasonably promptly after a share of Restricted Stock vests, the Company shall cause to be delivered to the Participant to whom such shares were granted a certificate evidencing such share, free of the legend set forth in Section 8(f) hereof, or cause a removal of such legend from the book entry evidencing such shares. | |
| (h) | Effect of Termination of Employment |
| (1) | Except as the Committee in its sole and absolute discretion may otherwise provide in the applicable Agreement, and subject to the Committee’s authority pursuant to Section 4 hereof, upon the termination of a Participant’s employment for any reason other than Cause, any and all shares to which restrictions on transferability apply shall be immediately forfeited by the Participant and transferred to, and reacquired by, the Company; provided that if the Committee, in its sole and absolute discretion, shall within thirty (30) days after such termination of employment notify the Participant in writing of its decision not to terminate the Participant’s rights in such shares, then the Participant shall continue to be the owner of such shares subject to such continuing restrictions as the Committee may prescribe in such notice. In the event of a forfeiture of shares pursuant to this section, the Company shall repay to the Participant (or the Participant’s estate) any amount paid by the Participant for such shares. In the event that the Company requires a return of shares, it shall also have the right to require the return of all dividends paid on such shares, whether by termination of any escrow arrangement under which such dividends are held or otherwise. | |
| (2) | In the event of the termination of a Participant’s employment for Cause, all shares of Restricted Stock granted to such Participant which have not vested as of the date of such termination shall immediately be returned to the Company, together with any dividends paid on such shares, in return for which the Company shall repay to the Participant any amount paid by the Participant for such shares. | |
| (i) | Effect of Change in Control |
| The Committee in its sole and absolute discretion may provide, either at the time of grant or thereafter, that upon the occurrence of a Change in Control, shares of Restricted Stock which have not theretofore vested shall immediately vest in whole or in part and all restrictions on such shares shall immediately lapse in whole or in part. |
122 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
9. Phantom Stock
| (a) | Vesting Date |
| Subject to Section 31 hereof, at the time of the grant of shares of Phantom Stock, the Committee shall establish a Vesting Date or Vesting Dates with respect to such shares. The Committee may divide such shares into classes and assign a different Vesting Date for each class. Provided that all conditions to the vesting of a share of Phantom Stock imposed pursuant to Section 9(c) hereof are satisfied, and except as provided in Section 9(d) hereof, upon the occurrence of the Vesting Date with respect to a share of Phantom Stock, such share shall vest. | |
| (b) | Benefit Upon Vesting |
| Upon the vesting of a share of Phantom Stock, the Participant shall be entitled to receive, within thirty (30) days of the date on which such share vests, an amount, in cash and/or shares of Company Stock, as determined by the Committee, equal to the sum of (1) the Fair Market Value of a share of Company Stock on the date on which such share of Phantom Stock vests, and (2) the aggregate amount of cash dividends paid with respect to a share of Company Stock during the period commencing on the date on which the share of Phantom Stock was granted and terminating on the date on which such share vests (unless such dividends have already been paid to the Participant). | |
| (c) | Conditions to Vesting |
| Subject to Section 31 hereof, at the time of the grant of shares of Phantom Stock, the Committee may impose such restrictions or conditions to the vesting of such shares as it, in its absolute discretion, deems appropriate, to be contained in the Agreement. | |
| (d) | Effect of Termination of Employment |
| Except as the Committee in its sole and absolute discretion may otherwise provide in the applicable Agreement, and subject to the Committee’s authority pursuant to Section 4 hereof, shares of Phantom Stock that have not vested, together with any dividends credited on such shares, shall be forfeited upon the Participant’s termination of employment for any reason. | |
| (e) | Effect of Change in Control |
| The Committee in its sole and absolute discretion may provide, either at the time of grant or thereafter, that upon the occurrence of a Change in Control, outstanding shares of Phantom Stock which have not theretofore vested shall immediately vest in whole or in part and payment in respect of such vested shares shall be made in accordance with the terms of this Plan. |
10. Stock Bonuses
In the event that the Committee grants a Stock Bonus, a certificate for the fiscal year ended Decembershares of Company Stock constituting such Stock Bonus shall be issued in the name of the Participant to whom such grant was made and delivered to such Participant as soon as practicable after the date on which such Stock Bonus is payable.
11. Other Awards
Other forms of Awards (“Other Awards”) valued in whole or in part by reference to, or otherwise based on, Company Stock may be granted either alone or in addition to other Awards under the Plan. Subject to the provisions of the Plan, the Committee shall have sole and complete authority to determine the persons to whom and the time or times at which such Other Awards shall be granted, the number of shares of Company Stock to be granted pursuant to such Other Awards and all other conditions of such Other Awards, subject to Section 31 2015 included in its Annual Report on Form 10-K forhereof.
12. Additional Provisions Regarding Nonemployee Director Awards
In addition to the year ended December 31, 2015).other applicable provisions of the Plan, the provisions of this Section 12 shall apply to Awards to Nonemployee Directors.
| (a) | General |
| Nonemployee Directors shall be eligible to receive Awards under the Plan (other than Incentive Stock Options). |
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 123 / 123 |
A-2
Appendix A Note Regarding Forward-Looking Statements and Non-GAAP Financial Measures
|
|
|
|
OTHER MATTERS / APPENDIX B
| (b) | Timing and Amount of Grant |
| Subject to Section 12(f) hereof, at such times as the Committee may determine, each then serving Nonemployee Director shall be granted an Award of such type and size and with such terms and conditions (consistent with the Plan) as the Committee in its sole and absolute discretion may determine. | |
| (c) | [Reserved.] |
| (d) | Term and Exercisability |
| Each Award granted to a Nonemployee Director shall become vested on such terms and conditions (consistent with the Plan) as the Committee in its sole and absolute discretion may determine. The vesting of each Award granted to a Nonemployee Director shall be fully accelerated upon a Change in Control. | |
| (e) | Termination |
| Except as the Committee in its sole and absolute discretion may otherwise provide in an applicable Agreement, and subject to the Committee’s authority pursuant to Section 4 hereof, in the event of the termination of a Nonemployee Director’s service with the Company, any outstanding and unvested Award held by such Nonemployee Director granted under the Plan shall expire at the commencement of business on the date of such termination. For purposes of the Plan, any termination of a Nonemployee Director’s service with the Company shall not be deemed to occur if the Nonemployee Director continues to serve for the Company as consultant, employee or in any other capacity. | |
| (f) | Limitations on Compensation of Nonemployee Directors and Chairman of Board of Directors |
| (1) | Except as provided in the last sentence of this Section 12(f)(1) and in Sections 12(f)(2) and 12(f)(3) hereof, during any calendar year, the aggregate number of shares of Company Stock subject to one or more Awards granted to a Nonemployee Director in such year shall not exceed 12,750 shares of Company Stock (calculated as described in Section 12(g) hereof). Notwithstanding the foregoing, except as provided in Section 12(f)(3) hereof, during the first calendar year in which a Nonemployee Director serves on the Board of Directors, the aggregate number of shares of Company Stock subject to one or more Awards granted to such Nonemployee Director in such year shall not exceed 34,000 shares of Company Stock (calculated as described in Section 12(g) hereof). | |
| (2) | Except as provided in Section 12(f)(3) hereof, during any calendar year, the aggregate number of shares of Company Stock subject to one or more Awards granted to a Nonemployee Director then serving as Chairman of the Board of Directors in such year shall not exceed 25,500 shares of Company Stock (calculated as described in Section 12(g) hereof). Notwithstanding the foregoing, except as provided in Section 12(f)(3) hereof, during the first calendar year in which a Nonemployee Director serves as Chairman of the Board of Directors, the aggregate number of shares of Company Stock subject to one or more Awards granted to such Nonemployee Director in such year shall not exceed 68,000 shares of Company Stock (calculated as described in Section 12(g) hereof). | |
| (3) | Notwithstanding the foregoing, any equity compensation of the Nonemployee Directors and the Chairman of the Board of Directors shall be, for the years set forth in the Settlement (as defined below), subject to the terms of the Stipulation of Compromise and Settlement, dated as of October 5, 2018, by and among the Plaintiffs and the Individual Defendants (each as defined therein) and the Company, as nominal defendant (the “Settlement”) in the shareholder derivative actions entitledCement Masons Local 780 Pension Fund, et ano. v. Leonard S. Schleifer, et al., Index No. 654453/2015 (N.Y. Co.) (filed December 30, 2015) andPublic Employees’ Retirement System of Mississippi v. Leonard S. Schleifer, et al., Index No. 656813/2017 (N.Y. Co.) (filed November 8, 2017). | |
| (g) | Calculation of Limitations on Nonemployee Director Compensation |
| For purposes of applying the limits on the Awards to Nonemployee Directors specified in Sections 12(f)(1) and 12(f)(2) hereof, (1) an Award other than a Nonqualified Stock Option or Stock Appreciation Right shall be treated as an Award of one and one-half (1.5) shares of Company Stock for each share actually subject to such Award and (2) a Nonqualified Option or Stock Appreciation Right shall be treated as an Award of one share of Company Stock for each share actually subject to such Award. |
124 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
13. Rights as a Shareholder
No person shall have any rights as a shareholder with respect to any shares of Company Stock covered by or relating to any Award until the date of issuance of a stock certificate with respect to such shares or registering a book entry evidencing the issuance of such shares. Except as otherwise provided in Section 3(c) hereof, no adjustment to any Award shall be made for dividends or other rights for which the record date occurs prior to the date such stock certificate is issued or such book entry is registered.
14. No Employment Rights; No Right to Award
Nothing contained in the Plan or any Agreement shall confer upon any Participant any right with respect to the continuation of employment by the Company or interfere in any way with the right of the Company, subject to the terms of any separate employment agreement to the contrary, at any time to terminate such employment or to increase or decrease the compensation of the Participant.
No person shall have any claim or right to receive an Award hereunder. The Committee’s granting of an Award to a participant at any time shall neither require the Committee to grant any other Award to such Participant or other person at any time or preclude the Committee from making subsequent grants to such Participant or any other person.
15. Securities Matters
| (a) | The Company shall be under no obligation to effect the registration pursuant to the Securities Act of any interests in the Plan or any shares of Company Stock to be issued hereunder or to effect similar compliance under any state laws. Notwithstanding anything herein to the contrary, the Company shall not be obligated to cause to be issued or delivered any certificates, or to cause to be registered any book entries, evidencing shares of Company Stock pursuant to the Plan unless and until the Company is advised by its counsel that the issuance and delivery of such certificates, or the book-entry registration, is in compliance with all applicable laws, regulations of governmental authority and the requirements of any securities exchange on which shares of Company Stock are traded. The Committee may require, as a condition of the issuance and delivery of certificates, or the book-entry registration, evidencing shares of Company Stock pursuant to the terms hereof, that the recipient of such shares make such agreements and representations, and that such certificates or book entries bear or are subject to such legends, as the Committee, in its sole discretion, deems necessary or desirable. |
| (b) | The transfer of any shares of Company Stock hereunder shall be effective only at such time as counsel to the Company shall have determined that the issuance and delivery of such shares is in compliance with all applicable laws, regulations of governmental authority and the requirements of any securities exchange on which shares of Company Stock are traded. The Committee may, in its sole discretion, defer the effectiveness of any transfer of shares of Company Stock hereunder in order to allow the issuance of such shares to be made pursuant to registration or an exemption from registration or other methods for compliance available under federal or state securities laws. The Committee shall inform the Participant in writing of its decision to defer the effectiveness of a transfer. During the period of such deferral in connection with the exercise of an Option, the Participant may, by written notice, withdraw such exercise and obtain the refund of any amount paid with respect thereto. |
16. Withholding Taxes
Whenever cash is to be paid pursuant to an Award, the Company shall have the right to deduct therefrom an amount sufficient to satisfy any federal, state and local withholding tax requirements related thereto.
Whenever shares of Company Stock are to be delivered pursuant to an Award, the Company shall have the right to require the Participant to remit to the Company in cash an amount sufficient to satisfy any federal, state and local withholding tax requirements related thereto. With the approval of the Committee, a Participant may satisfy the foregoing requirement by electing to have the Company withhold from delivery shares of Company Stock having a value up to the maximum amount of tax required to be withheld. Such shares shall be valued at their Fair Market Value on the date of which the amount of tax to be withheld is determined. Fractional share amounts shall be settled in cash. Such a withholding election may be made with respect to all or any portion of the shares to be delivered pursuant to an Award.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 125 / 125 |
OTHER MATTERS / APPENDIX B
17. Notification of Election Under Section 83(b) of the Code
If you would likeany Participant shall, in connection with the acquisition of shares of Company Stock under the Plan, make the election permitted under Section 83(b) of the Code, such Participant shall notify the Company of such election within ten (10) days of filing notice of the election with the Internal Revenue Service.
18. Notification Upon Disqualifying Disposition Under Section 421(b) of the Code
Each Agreement with respect to reducean Incentive Stock Option shall require the costs incurred by our companyParticipant to notify the Company of any disposition of shares of Company Stock issued pursuant to the exercise of such Option under the circumstances described in mailing proxy 1234567 VOTE BY MAIL 123,456,789,012.12345 TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: KEEP THIS PORTION FOR YOUR RECORDS DETACH AND RETURN THIS PORTION ONLY THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. Section 421(b) of the Code (relating to certain disqualifying dispositions), within ten (10) days of such disposition.
19. Amendment or Termination of the Plan
The Board of Directors recommends you vote FORmay, at any time, suspend or terminate the following: 1. Election of Directors Nominees For 0 0 0 For 0 Against 0 0 0 Against 0 Abstain 0 0 0 Abstain 0 01 Michael S. Brown 02 Leonard S. Schleifer 03 George D. Yancopoulos ThePlan or revise or amend it in any respect whatsoever; provided, however, that shareholder approval shall be required if and to the extent the Board of Directors recommends you vote FOR the following proposal: 2 Ratificationdetermines that such approval is appropriate for purposes of satisfying Section 422 of the appointmentCode or Rule 16b-3 or other applicable law or regulation (including listing standards). Awards may be granted under the Plan prior to the receipt of PricewaterhouseCoopers LLPsuch shareholder approval but each such grant shall be subject in its entirety to such approval and no award may be exercised, vested or otherwise satisfied prior to the receipt of such approval. Nothing herein shall restrict the Committee’s ability to exercise its discretionary authority pursuant to Section 4 hereof, which discretion may be exercised without amendment to the Plan. Without limiting the authority of the Committee under Section 3(c) hereof or Section 3(d) hereof, and subject to Section 32 hereof, no action hereunder may, without the consent of a Participant, reduce the Participant’s rights under any outstanding Award.
20. Transferability
Upon the death of a Participant, outstanding Awards granted to such Participant may be exercised only by the executor or administrator of the Participant’s estate or by a person who shall have acquired the right to such exercise by will or by the laws of descent and distribution. No transfer of an Award by will or the laws of descent and distribution shall be effective to bind the Company unless the Committee shall have been furnished with (a) written notice thereof and with a copy of the will and/or such evidence as the Company's independent registered public accounting firmCommittee may deem necessary to establish the validity of the transfer, and (b) an agreement by the transferee to comply with all the terms and conditions of the Award that are or would have been applicable to the Participant and to be bound by the acknowledgments made by the Participant or in connection with the grant of the Award.
During the lifetime of a Participant, the Committee may, in its sole and absolute discretion, permit the transfer of an outstanding Option, unless such Option is an Incentive Stock Option and the Committee and the Participant intends that it shall retain such status. Subject to the approval of the Committee and to any conditions that the Committee may prescribe, a Participant may, upon providing written notice to the Secretary of the Company, elect to transfer any or all Options granted to such Participant pursuant to the Plan to members of his or her immediate family (including, but not limited to, children, grandchildren and spouse or to trusts for the fiscal year ending December 31, 2016. NOTE: benefit of such immediate family members or to partnerships in which such family members are the only partners) or to other persons or entities approved by the Committee; provided, however, that no such transfer by any Participant may be made in exchange for consideration.
21. Expenses
The expenses of the Plan shall be paid by the Company.
22. Failure to Comply
In addition to the remedies of the Company elsewhere provided for herein, failure by a Participant (or beneficiary) to comply with any of the terms and conditions of the Plan or the applicable Agreement, unless such failure is remedied by such Participant (or beneficiary) within ten days after notice of such failure by the Committee, shall be grounds for the cancellation and forfeiture of such Award, in whole or in part, as the Committee, in its absolute discretion, may determine.
126 /  | 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |
OTHER MATTERS / APPENDIX B
23. Effective Date and Term of Plan
The Plan shall be subject to the requisite approval of the shareholders of the Company. Unless earlier terminated by the Board of Directors, the right to grant Awards under the Plan shall terminate on the tenth anniversary of the Effective Date. Awards outstanding at Plan termination shall remain in effect according to their discretion,terms and the named proxiesprovisions of the Plan.
24. Applicable Law
Except to the extent preempted by any applicable federal law, the Plan shall be construed and administered in accordance with the laws of the State of New York without reference to its principles of conflicts of law.
25. Participant Rights
No Participant shall have any claim to be granted any award under the Plan, and there is no obligation for uniformity of treatment for Participants. Except as provided specifically herein, a Participant or a transferee of an Award shall have no rights as a shareholder with respect to any shares covered by any Award until the date of the issuance of a Company Stock certificate to him or her or the book-entry registration in such Participant’s or such transferee’s name for such shares.
26. Unfunded Status of Awards
The Plan is intended to constitute an “unfunded” plan for incentive and deferred compensation. With respect to any payments not yet made to a Participant pursuant to an Award, nothing contained in the Plan or any Agreement shall give any such Participant any rights that are greater than those of a general creditor of the Company.
27. No Fractional Shares
No fractional shares of Company Stock shall be issued or delivered pursuant to the Plan. The Committee shall determine whether cash, other Awards, or other property shall be issued or paid in lieu of such fractional shares or whether such fractional shares or any rights thereto shall be forfeited or otherwise eliminated.
28. Beneficiary
A Participant may votefile with the Committee a written designation of a beneficiary on such other mattersform as may properly come beforebe prescribed by the meetingCommittee and may, from time to time, amend or revoke such designation. If no designated beneficiary survives the Participant, the executor or administrator of the Participant’s estate shall be deemed to be the grantee’s beneficiary.
29. Substitute Awards
Notwithstanding any adjournment(s)other provision of the Plan, the terms of Substitute Awards may vary from the terms set forth in the Plan to the extent the Committee deems appropriate to conform, in whole or postponement(s) thereof. John Sample attorney, executor, administrator,in part, to the provisions of the awards in substitution for which such Substitute Awards are granted.
30. Interpretation
The Plan is designed and intended to permit the grant of Awards which comply with the exemption set forth in Rule 16b-3 and to permit the grant of awards which either comply with or exempt from the provisions of Section 409A of the Code. All provisions hereof shall be construed in a manner to so comply with such intention.
31. Severability
If any provision of the Plan is held to be invalid or unenforceable, the other fiduciary, please give full ANY CITY, ON A1A 1A1 partnership name, by authorized officer. Signature [PLEASE SIGN WITHIN BOX] Date Signature (Joint Owners) Date 02 0000000000 1 OF 1 1 2 0000282794_1 R1.0.1.25 Please sign exactlyprovisions of the Plan shall not be affected but shall be applied as your name(s) appear(s) hereon. When signingif the invalid or unenforceable provision had not been included in the Plan.
32. Applicability of Company Policy
By accepting an Award under the Plan, each Participant agrees that the terms and conditions of the Company’s Policy Regarding Recoupment or Reduction of Incentive Compensation for Compliance Violations, as title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or Investor Address Line 1 Investor Address Line 2 Investor Address Line 3 Investor Address Line 4 Investor Address Line 5 1234 ANYWHERE STREET SHARES CUSIP # JOB #SEQUENCE # effect from time to time, shall apply to such Participant’s Award(s) under the Plan.
| 2020 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  / 127 / 127 |


777 OLD SAW MILL RIVER ROAD
TARRYTOWN, NY 10591-6707
REGENERON PHARMACEUTICALS, INC.
777 OLD SAW MILL RIVER ROAD
TARRYTOWN, NY 10591-6707
ATTN: CORPORATE SECRETARY
VOTE BY INTERNET
Before The Meeting - Go towww.proxyvote.com
Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M.p.m. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. ELECTRONIC DELIVERY OF FUTURE PROXY MATERIALS materials, you can consent
During The Meeting - Go to receiving all future proxy statements, proxy cards and annual reports electronicallywww.virtualshareholdermeeting.com/REGN2020
You may attend the meeting via e-mail or the Internet. To sign up for electronic delivery, please follow the instructions above to vote using the Internet and when prompted, indicatevote during the meeting. Have the information that you agree to receive or access proxy materials electronicallyis printed in future years. the box marked by the arrow available and follow the instructions.
VOTE BY PHONE - 1-800-690-6903
Use any touch-tone telephone to transmit your voting instructions up until 11:59 John Sample 23456 7P.M.p.m. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you call and then follow the instructions. 1234567
VOTE BY MAIL
Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. NAME THE COMPANY NAME INC. - COMMON THE COMPANY NAME INC. - CLASS A THE COMPANY NAME INC. - CLASS B THE COMPANY NAME INC. - CLASS C THE COMPANY NAME INC. - CLASS D THE COMPANY NAME INC. - CLASS E THE COMPANY NAME INC. - CLASS F THE COMPANY NAME INC. - 401 K CONTROL # SHARES 123,456,789,012.12345 123,456,789,012.12345 123,456,789,012.12345 123,456,789,012.12345 123,456,789,012.12345 123,456,789,012.12345 123,456,789,012.12345 x PAGE 1 OF 2 REGENERON PHARMACEUTICALS, INC. 777 OLD SAW MILL RIVER ROAD TARRYTOWN, NY 10591-6707 Investor Address Line 1 Investor Address Line 2 Investor Address Line 3 Investor Address Line 4 Investor Address Line 5 8 8 8 1 1234 ANYWHERE STREET ANY CITY, ON A1A 1A1 234567 234567 234567 234567
| TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: | |
| D13186-P35010-Z76486 | KEEP THIS PORTION FOR YOUR RECORDS |
| DETACH AND RETURN THIS PORTION ONLY |
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED.

| REGENERON PHARMACEUTICALS, INC. | |||||||||||||||||
| The Board of Directors recommends you vote FOR the following proposals: | |||||||||||||||||
| 1. | Election of Directors | ||||||||||||||||
| Nominees: | For | Against | Abstain | ||||||||||||||
| 1a. | N. Anthony Coles, M.D. | o | o | o | |||||||||||||
| 1b. | Joseph L. Goldstein, M.D. | o | o | o | |||||||||||||
| 1c. | Christine A. Poon | o | o | o | |||||||||||||
| 1d. | P. Roy Vagelos, M.D. | o | o | o | |||||||||||||
| 1e. | Huda Y. Zoghbi, M.D. | o | o | o | For | Against | Abstain | ||||||||||
| 2. | Ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2020. | o | o | o | |||||||||||||
| 3. | Proposal to approve the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. | o | o | o | |||||||||||||
| 4. | Proposal to approve, on an advisory basis, executive compensation. | o | o | o | |||||||||||||
| NOTE:Such other business as may properly come before the meeting or any adjournment thereof. | |||||||||||||||||
| Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. | |||||||||||||||||
| Signature [PLEASE SIGN WITHIN BOX] | Date | Signature (Joint Owners) | Date | |||||||||||
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting:
The Notice &and Proxy Statement and Annual Report is/ are available at www.proxyvote.com www.proxyvote.com.
| D13187-P35010-Z76486 |
REGENERON PHARMACEUTICALS, INC.
Annual Meeting of Shareholders
June 10, 201612, 2020 10:30 AM
This proxy is solicited by the Board of Directors
The undersigned hereby appointsappoint(s) Leonard S. Schleifer, M.D., Ph.D. and Joseph J. LaRosa, and each of them individually, as lawful proxies, each with full power of substitution, to represent the undersigned, with all powers that the undersigned would possess if personally present, and to vote, as indicated on the reverse side of this card, all of the shares of Common Stock and Class A Stock of Regeneron Pharmaceuticals, Inc.REGENERON PHARMACEUTICALS, INC. that the undersigned would be entitled to vote if personally present at the Annual Meeting of Shareholders of the Company to be held on June 10, 201612, 2020 live via webcast at www.virtualshareholdermeeting.com/REGN2020 and in person at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York 10591 or at any adjourned or postponed session thereof. This proxy revokes all prior proxies given by the undersigned.
SHARES REPRESENTED BY THIS PROXY WILL BE VOTED AT THE MEETING AND AT ANY ADJOURNMENTS OR POSTPONEMENTS THEREOF IN ACCORDANCE WITH THE SHAREHOLDER'SSHAREHOLDER’S SPECIFICATIONS ABOVE.ON THE REVERSE. IF YOU SIGN AND RETURN YOUR PROXY CARD IN A TIMELY MANNER, BUT DO NOT INDICATE HOW THESE SHARES ARE TO BE VOTED, THIS PROXY WILL BE VOTED FOR THE ELECTION OF THE NOMINEES NAMED ABOVEON THE REVERSE AS DIRECTORS AND FOR PROPOSAL 2.PROPOSALS 2, 3 AND 4. THIS PROXY ALSO CONFERS DISCRETIONARY AUTHORITY WITH RESPECT TO MATTERS NOT KNOWN OR DETERMINED AT THE TIME OF THE MAILING OF THE NOTICE OF THE ANNUAL MEETING OF SHAREHOLDERS TO THE UNDERSIGNED.
Continued and to be signed on reverse side 0000282794_2 R1.0.1.25